A Survey on the Status of Pangolins By Camera Trapping in Deng-Deng National Park, Eastern Region, Cameroon
MELLE EKANE MAURICE
Affiliation
1Department of Environmental Science, University of Buea, Cameroon
2Conservation Action Research Network (CARN) for the Congo Basin, Central African Region
Corresponding Author
Melle Ekane Maurice, Department of Environmental Science, University of Buea, P.O. Box 63, Cameroon; E-mail: melleekane@gmail.com
Citation
Maurice, M.E., et al. A Survey on the Status of Pangolins by Camera Trapping in Deng-Deng National Park, Eastern Region, Cameroon. (2019) J Environ Health Sci 5(1): 40-46.
Copy rights
© 2019 Maurice, M.E. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Camera-trap; Wildlife species; Pangolins; National park; Wildlife population
Abstract
Information on the distribution of wildlife species is important in prescribing sound management practices for a protected area. Despite the efficiency and flexibility of modern camera-traps, they cannot observe every species with equal precision. For one, it can be difficult to get an accurate count of population abundance. Camera-trapping systems are not the only solution to wildlife research inventory, but they are a useful tool in creating understanding on the presence and population of wildlife species, especially the nocturnal animals in the wild. Camera-taps offer a way to answer questions about wildlife population, besides population density. The determination of pangolins status was established in Deng-Deng national park by the use of camera-trapping system. A total of 15 camera-traps (10 Cuddle backs and 5 Bushnells) were set in order to confirm the presence of pangolins in the area. These camera-traps were set in different locations of the national park area to record the presence of wildlife species, especially the pangolins. The retrieval of the camera-traps after a period of two months identified the existence of three species of pangolins; giant ground pangolin (Smutsia gigantean), white-bellied pangolin (Phataginus tricuspis), and black-bellied pangolins (Phataginus tetradactyla). A total number of 41 pangolin images were caught by the cameras within the study period. The results have shown that cameras mounted in the mixed liana forest area in the national park recorded the highest frequency of pangolin images 25.33%. Also, the swamp forest vegetation witnessed the least frequency of pangolin endemism 1.33% in the national park. In addition, the study revealed that the pangolin species distribution is significantly associated to forest vegetation type and the presence of human signs, χ2= 22.675 df = 14 P > 0.05, (fig.3) and χ2 = 5.004 df = 14 P < 0.05 (fig.4) respectively. The use of wildlife camera-trapping system remains one of the best methods for pangolin population distribution survey in protected areas.
Introduction
The distribution knowledge of species and occurrence is crucial for planning and evaluating conservation strategies (Tobler et al., 2008). The methods commonly used for mammal inventories include line transects (Plumptre, 2000), direct counts (Silveira, Ja′como & Filho, 2003), indirect evidence, for example, nests, tracks and signs (Plumptre & Reynolds, 1997), trapping (Kasangaki, Kityo & Kerbis, 2003), interviews with local people (Andama, 2000a) and camera-trapping (Tobler et al. 2008). Camera-traps have become increasingly popular as technology has improved and costs have decreased (Yasuda, 2004; Tobler et al., 2008). Camera-traps have been used to estimate mammal density (Kelly et al., 2008; Maffei & Noss, 2008), to determine animal activity patterns (Azlan & Lading, 2006; Azlan & Sharma, 2006), to estimate species richness (O’Brien, Kinnaird & Wibisono, 2010), to estimate community structure and diversity (Ahumada et al., 2011) and to detect species presence (Giman et al., 2007; Tobler et al., 2008).
The Zoological Society of London (ZSL) is currently using diurnal line transects, camera-traps, and nocturnal searches to assess the populations of the sunda pangolins (Manis javanica) in the Salakpra Conservation Landscape and the Khlong Nakha Wildlife Sanctuary, Thailand (ZSL, 2017). Wilcox et al. (2011) have used diurnal searches for tracks and signs and nocturnal spotlighting walks in small carnivores and pangolins (Sunda pangolins and Chinese pangolins) surveys in Vietnam. In a study carried out by Former Mentor-POP, (2017) in the Campo Ma’an national park indicated that line transects were established in two areas distinguished by their assumed level of hunting pressure based upon their distance from the nearest human settlements. They assumed that, in areas near villages hunting pressure on pangolins were greater (Abernethy et al. 2013). In each forest category, they established at least fifteen 1km long line transects at a distance of 250m apart. Along each transect and with the help of local guides, a 100% search and recording of all signs indicating pangolin presence was conducted. Targeted signs included: sighting of the animal, pangolin burrows, feeding remains, foot-print, and feces. When a sign was seen, two local guides observed the signs critically and if they both agreed it was a pangolin sign, the sign was recorded (Mentor-POP Report, 2017).
It should be noted that most studies carried out on pangolin especially those pertaining to its conservation status has pointed that the high demand of pangolin scales for Asian medicines has been a major pushing factor to pangolin illegal hunting and trafficking. The steep rise in demand for pangolin scales driven by traditional remedies in Asia has greatly increased black market prices and is now driving intensive commercial hunting of all pangolin species in Africa (Challender & Hywood, 2012; Cheng, Zing, & Bonebrake, 2017). Local and international trade is another major threat that has greatly endangered the species causing it to be under severe hunting pressure due to local consumption and rising demand for its scales and meat in east Asian markets, especially the Chinese markets (Baillie et al., 2015; Challender, 2011). The pangolin population in India and Pakistan for example has been over-exploited for international trade with most smuggled specimens and parts ending up in Chinese and Myanmar markets (Baillie et al., 2015). The presumable decline in pangolin population in the wild is a conservation disturbance; hence Cameroon government has encouraged research in the area of its population status. The pangolin species and population knowledge in Deng-Deng national park in the eastern region of Cameroon is absent, like in most of the other protected areas in the country. The camera-trapping method was considered in this study as the most reliable and effective tool to use in surveying the presence of this endangered animal species in the national park.
Materials and Methods
Description of the Study Area
The Deng-Deng National Park is found in the east region of Cameroon and covers a surface area of approximately 68,264 hectares. It is located between 5° 21’ 2.8” N to Longitude 13° 26’ 30.4” E (Maisels et al., 2010a). It has mean monthly daily temperatures of about 34oc during the dry season. During the wet season, temperature falls to a daily mean of about 22oC, with the peak of rainfall recorded at about 2500mm in August (Maisels et al., 2010a). The park has savanna ecosystem and forest vegetation found at the northern and southern portions of the park. The national park equally has diverse species of wildlife population, such as gorillas, chimpanzees, monkeys, antelopes, golden cats, porcupines, squirrels, red river hogs, duikers, and several others. This has made the park an attractive zone for research and exploration (Maisels et al., 2010a).

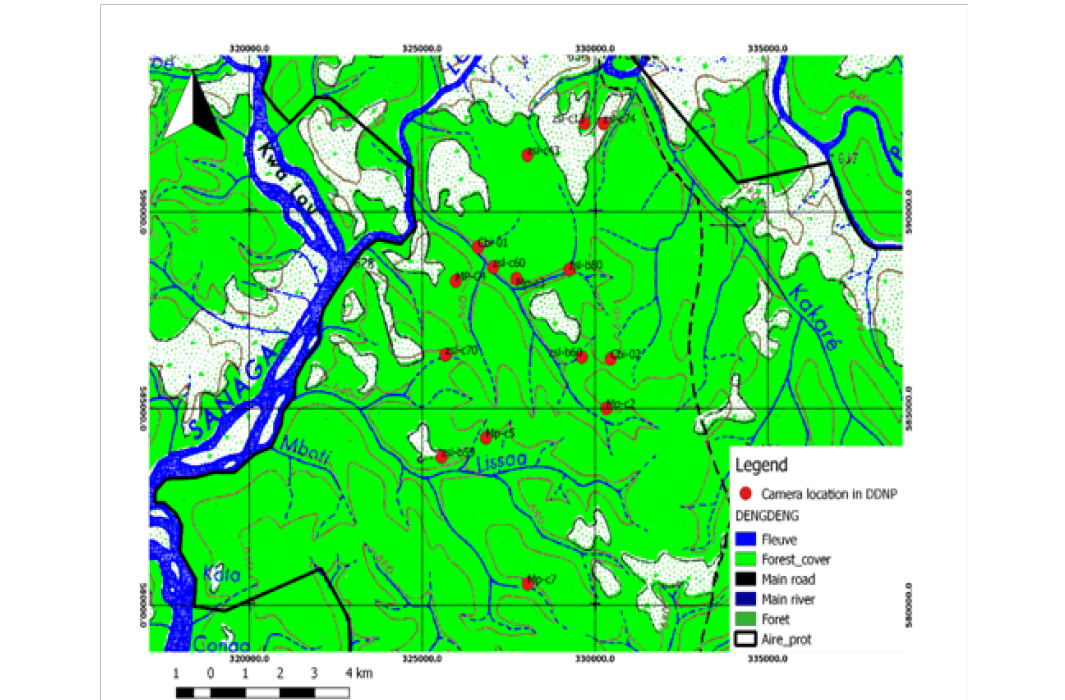
Figure 1: Location of cameras in the Deng-Deng national park
Camera-traps setting
Only the feeding points on dead woods that appeared to be widely used by pangolins as a feeding site and as pathways were chosen for the mounting of cameras in addition to a few suspected burrows. A total of 15 camera-traps (10 Cuddle backs and 5 Bushnells) were mounted in order to confirm the presence of pangolins in the area. According to Ancrenaz (2012), setting the cameras so that the sensor is 30-40 cm above the ground gives good results for small carnivores because higher heights camera settings may not record small mammals. In an area showing potential signs of pangolins, a tree was chosen to provide the camera with a suitable orientation ensuring that the camera is not facing west or east as sunlight would affect pictures at sunrise and sunset. The vegetation was cleared in front of the camera to avoid obscureness. The cameras were positioned perpendicular to the natural assumed pangolin sign at a distance of 4-8m with the aim of obtaining full body lateral images of the animals. The digital camera was often used to check that the ground surface in front of the Camera was visible to a distance of 4 metres away and when necessary a small stick was used to ensure the camera is angled appropriately. After installing a camera a detection test was conducted to ensure correct operation of the camera, the animal movement was simulated at this spot by the research team to test the effectiveness of the camera. Related information on the environmental characteristics such as the forest-type and the distance to the nearest water point were also recorded for each camera.
The cameras installed in Deng-Deng national park were retrieved after two months, and images of wildlife such as the pangolins and other wildlife species were observed. In Africa, Akpona et al. (2008) have used strip transects (3 km x 1 km each) to assess the density of the white-bellied pangolin (Phataginus tricuspis) in the Lama Forest Reserve, Benin. Camera-traps were installed in order to confirm pangolin presence with images. The camera-traps were installed in areas with abundant fresh or active sign of pangolin. Cameras were placed at a height of 30 cm above the ground.
Today, camera-traps are cheap enough for wildlife enthusiasts to buy, and are popular with hunters for determining where their targets are on the landscape. Aided by this wide market popularity, camera makers have made strides in their functionality and simplicity of use over the last two decades. Most are self-contained, their sensors, flashes, and control systems are all in one package, which simplifies deployment and makes them weather proof and durable. Instead of trip-wires, most wildlife researchers use passive infrared sensors to detect when something warmer than the surrounding environment is moving around the camera. Their flashes can be white, to produce clear images of wildlife markings, or infrared, which can only be seen by some mammals, to reduce disturbance. Some can even record video to capture behavior, but this drains batteries and fills memory cards quicker than photos (Rovero et al. 2013).
Results
The survey has shown that 12 cameras out of 15 captured the images of 41 pangolins (Tab. 4.2). Each of the 12 cameras captured at least the image of one pangolin. Also, from the cameras, different pangolin species were identified in this national park. Pangolins are highly endangered due to high poaching pressure, for this reason their population is drastically declining from the wild. Formerly, the pangolins like any other wildlife species were poached by the local population for consumption, till the recent high demand of their scales and other body parts for traditional medicine in Asia and Africa. It is believed that most of the pangolin poaching today is for the commercializing of the scales, a source income generation for the local population.
Table 1: Camera setting sites
|
Camera-type |
Forest-Type |
Sites or Sections |
Type of Activity |
Number of pangolin picture |
|
Mp-C2 |
FML |
Fallen tree-trunk |
good |
2 |
|
Cbi-02 |
FML |
Fallen tree-trunk |
good |
7 |
|
Zsl-b60 |
FM |
Tree-trunk |
good |
2 |
|
Mp-c3 |
FML |
Tree-trunk |
good |
3 |
|
Zsl-C70 |
FMSO |
Tree-hollow |
good |
0 |
|
Mp-C5 |
FML |
Tree trunk-hollow |
good |
2 |
|
Zsl-b59 |
FML |
Tree-hollow |
good |
0 |
|
Mp-C7 |
FM |
Tree-hollow |
good |
0 |
|
Mp-C4 |
FML |
Fallen tree-trunk |
good |
3 |
|
Cbi-01 |
FMSO |
Feeding ground burrow |
good |
2 |
|
Zsl-C60 |
FM |
Tree hollow |
good |
2 |
|
Zsl-b80 |
FMSO |
Ground feeding site |
good |
5 |
|
Zsl-C43 |
F-S |
Ground feeding site |
good |
2 |
|
Zsl-c13 |
FMSO |
Tree trunk with a nutrition site |
good |
11 |
|
Zsl-C74 |
FMSO |
Ground feeding site |
Good |
|
The results have shown that cameras in the mixed liana forest in the national park recorded the highest frequency of pangolin images 25.33% (fig. 2). A liana forested area provides a good protective cover for the pangolins for reasons that its vegetation density prevents poachers and other predators from accessibility. Secondly, some of these areas are also rich in dead wood materials that provide breeding places for ants like termites, a source of food to the pangolins population. The swamp-forest vegetation witnessed the least frequency of pangolin presence 1.33% in the national park.

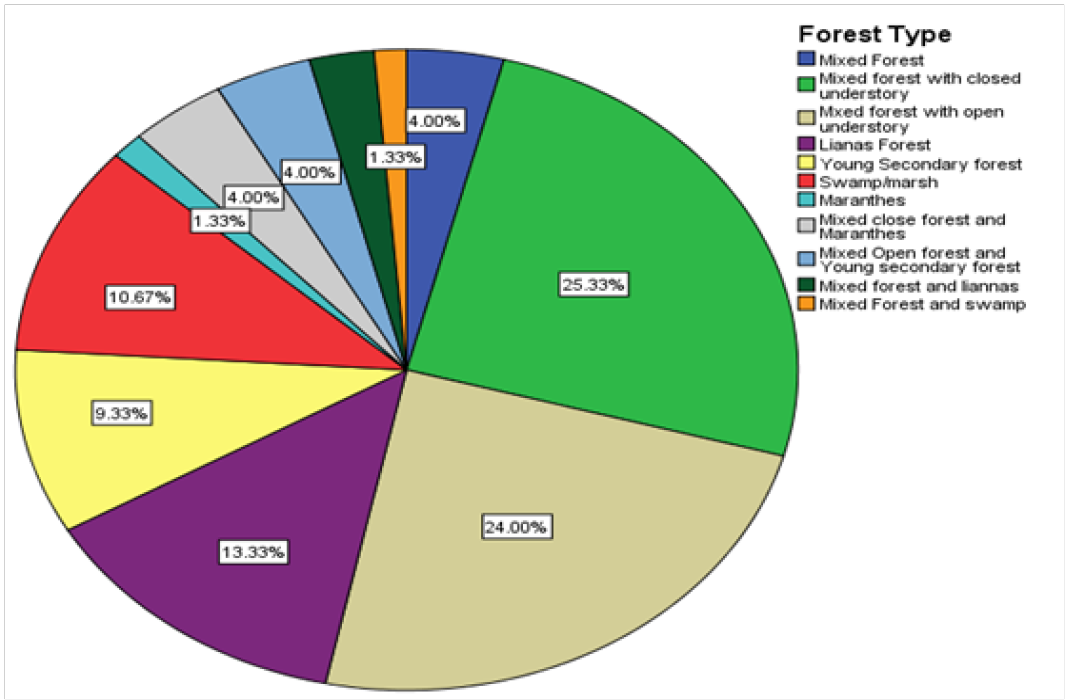
Figure 2: Forest type and pangolin population
Majority of the cameras installed in Deng-Deng national park successfully detected and took images of three pangolin species. The cameras confirmed the presence of pangolins in the park in various locations they were mounted. The nocturnal behavior of pangolins created some challenges in assessing its population in the mixed forest vegetation rainforest ecosystem. The use of wildlife camera-traps and the pangolin sign encounter rates examination remain one of the best methods for its population occurrence observation. During the transect-walk, the research team recorded 48.00% of the feeding sign encounter rates (tab.2). The frequency of foot-prints and living burrows recorded 5.30% each respectively. The frequency at which pangolin signs were encountered is a clear indication of pangolin occurrence in the national park.

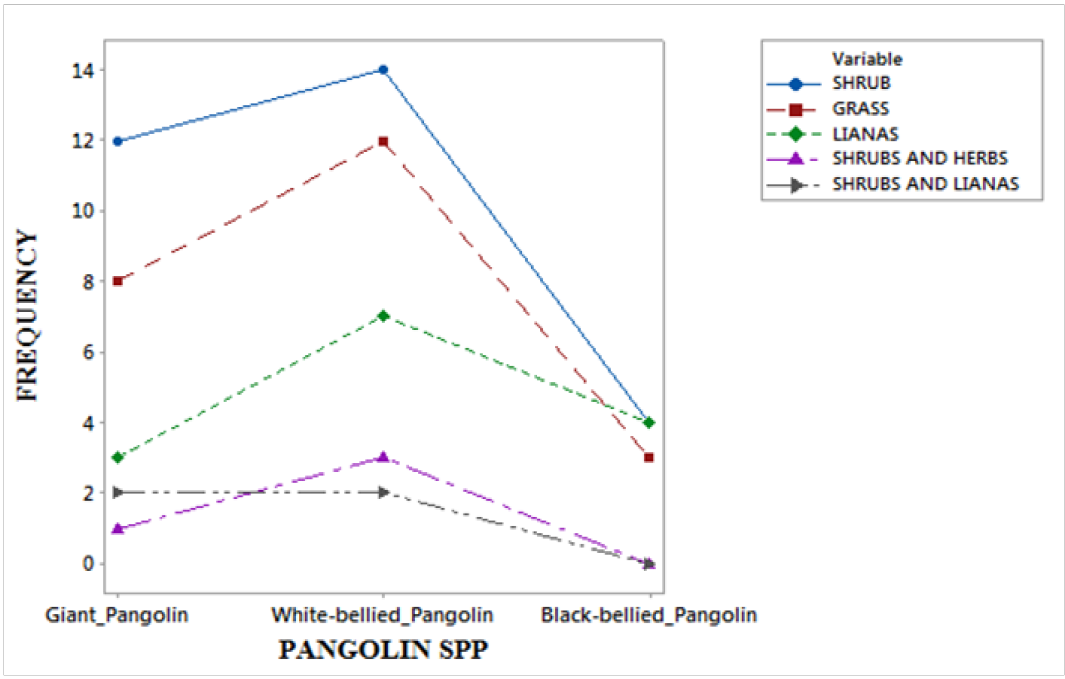
Figure 3: The forest vegetation type and Pangolin spp
Table 2: The encounter rates of pangolin signs
|
Animal Signs |
Total |
||||||||||
|
|
Feeding Burrow |
Trail (Footprint) |
Feeding Sign |
Path |
Direct Observation |
Direct Observation |
Living Burrow |
Track and feeding sign |
Path and living burrow |
None |
|
|
Cloudy |
Count |
4 |
0 |
12 |
2 |
0 |
3 |
1 |
2 |
1 |
25 |
|
% |
16.0% |
0.0% |
48.0% |
8.0% |
0.0% |
12.0% |
4.0% |
8.0% |
4.0% |
100.0% |
|
|
Sunny |
Count |
5 |
4 |
24 |
2 |
1 |
3 |
5 |
2 |
3 |
50 |
|
% |
10.0% |
8.0% |
48.0% |
4.0% |
2.0% |
6.0% |
10.0% |
4.0% |
6.0% |
100.0% |
|
|
Total |
count |
9 |
4 |
36 |
4 |
1 |
6 |
6 |
4 |
4 |
75 |
|
% |
12.0% |
5.3% |
48.0% |
5.3% |
1.3% |
8.0% |
8.0% |
5.3% |
5.3% |
100.0% |
|
The results have revealed that the pangolin species distribution is significantly associated to forest vegetation type and the presence of human signs, χ2= 22.675 df = 14 P > 0.05 (fig.3) and χ2 = 5.004 df = 14 P < 0.05 (fig.4) respectively. The distribution of the pangolins in the national park is neither affected by human presence nor the forest vegetation type. The slow moving and nocturnal animal species does not seem to consider humans as a potential threat to their existence in the wild, even as they are hunted. One of the survival strategies of wildlife in most areas is shyness from human presence or sight. However, the nocturnal character and smallness in body size of the pangolin species in Cameroon as compared to the duikers and other wildlife might help them survive night poaching in most cases. Some farmers in villages claimed they sometimes hand-pick the pangolins from their bush trails without a fight. However, most wild rodents are swift and are very ready to fight with bites when caught or threaten with a hand-catch by humans or other predators, with pangolins the case is seemly different.

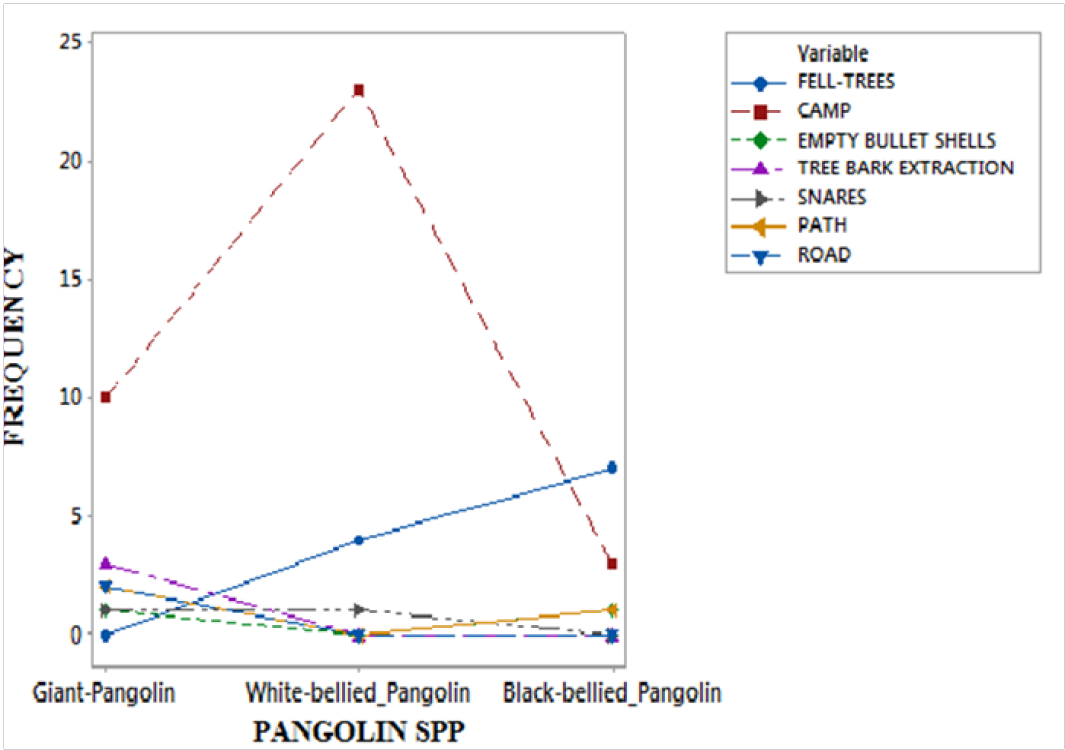
Figure 4: Human activity signs and the Pangolin spp
The presence of the termite mounds was observed in almost all the forest vegetation types, believed to be one of the reasons for their presence in these areas. Termite mounds are not only hiding places for pangolins but also serve as feeding sources, since they mostly feed on ants, specifically the termites. They are slow in movement and may not be good in covering distances for food; hence, the food availability is one of the determinants for a niche choice as other wildlife species.
Discussion
Camera-traps have become an important tool for monitoring rare, cryptic species in a wide range of environments (Cutler & Swann 1999). Even though camera-trapping for pangolins is a difficult task, this study has proven that the pangolins can be recorded with a camera-trap within 40-50 trapping nights using 12-15 camera-traps. It confirmed the presence of black-bellied pangolin (Phataginus tetradactyla), white-bellied pangolin (Phataginus tricuspis) and the giant pangolin (Smutsia gigantean) in Deng-Deng national park. This success has equally been recorded by the Zoological Society of London (ZSL) in Dja Biosphere Reserve using 30 camera-traps for over100 days (about 4000 trap nights) in 2016 (Bruce et al., 2017), where it recorded white-bellied pangolin (Phataginus tricuspis) and giant pangolin (Smutsia gigantean). Pangolins have also been recorded in camera-traps in other parts of Africa like South Sudan (Shreya, 2015) and Uganda (Treves et al., 2009). From informal discussions with a pangolin expert, a US Pangolin Researcher, who runs pangolin projects in the Republic of Cameroon, stated that the black-bellied pangolin is diurnal, rests mostly on tree tops and only descends when it feels uneasy. This might account for the absence of black-bellied pangolin in most camera-trap pictures because the camera-traps were set to primarily target ground species, making detection of arboreal pangolins difficult.
The strange behavior of the black-bellied pangolin was also confirmed by most local inhabitants in the Deng-Deng national park during the socio-economic surveys. Respondents said black-bellied pangolins (Phataginus tetradactyla) behave differently from giant and the white-bellied pangolins because it is very active during the day. Kingdon (2015) indicates that the black-bellied pangolin is active both during the day and during the night. In Asia, Marler (2016) recorded palawan pangolin (Manis culionensis) soon after camera-trap installation. Lim and Ng (2007) also photo-captured the sunda pangolin (Manis janica) soon after camera-traps were set, but were not successful observed in consecutive long-term photo captures of the animal, suggesting that pangolins were curious to find out what happened in the areas in which camera-traps were installed. This was however not the case in our study where pangolins were photo-captured over successive periods. Six pangolin independent photographic observations occurred within three days of camera installation, several other pangolin independent photographic observations after two weeks, and eight more pangolin independent photographic observations after one month. At the end of 60 nights of camera-trapping, there were a total of 41 photographs of pangolins. This suggests that African pangolins are not necessarily repelled or attracted by human presence, but continue operating normally under limited human presence. As expected, all the detections of pangolins were recorded at night, suggesting that the black-bellied, giant pangolin and the white-bellied pangolin are nocturnal (Kingdon, 2015). Our results suggest that tree pangolins or white bellied pangolins (Phataginus tricuspis) are more active after midnight as four of the five camera detections occurred between 10:00 PM and 5:00 AM. This is supported by other camera-trap surveys which reported that pangolin within the DBR had a peak of activity at 4 am (Bruce et al. 2017).
Pangolin direct counts are impractical and researchers may need to rely on indirect signs, such as tracks, scats or densites (Wilson and Delahay, 2001). Radio telemetry techniques are widely used in studying the ecology of wild animals as a cost-efficient method compared to GPS tracking methods. Such methods allow easy observation of the activity cycle, habitat preference, feeding grounds and resting places of the pangolins (Norman, 2009). Heath and Coulson, 1997 indicated that the potential of radio-tracking wild-caught pangolins to monitor home-range size and habitat utilization has been demonstrated by previous works. In the same study, Lim and Ng, (2008) and Richer et al., (1997), underscored the fact that radio transmitters were tagged to the dorsal scales closer to the base of the tail of pangolins to minimize disturbances to their usual behaviors in the wild. To that effect, they tested a field survey method to cost-effectively locate a pangolin in order to deploy a tracking tag by a research team. As camera-traps have been used previously to document elusive species (Whitworth, Braunholtz, et al. 2016), they placed camera-traps on potential pangolin burrows identified by local guides to test if it was possible to locate an active burrow. The camera-trapping techniques have also been used to determine the distribution and abundance of pangolins (Pabasara, 2016). In some cases, baits have been used to attract pangolins towards the camera-trap (Marler, 2016).
Conserving pangolins at their sources of extraction will require communities and officials to be aware of the importance and benefits of pangolin conservation so that they can be effectively involved in conservation measures based on participatory approaches (Chakkaravarthy, 2012). In Cameroon wildlife species are accorded various levels of protection through the 1994 Forestry and Wildlife Law. Wildlife animals are listed on classes A, B and C according their conservation status. Class A species are totally protected and cannot be hunted, captured, killed or traded while animals in class B could be hunted, captured or killed subject to a grant of a hunting permit. Class C species are partially protected and can be hunted, captured or killed as prescribed by the national government.
Conclusion
A comprehensive survey of nocturnal wildlife presence and population distribution in the wild is very challenging. Most of the nocturnal wildlife species are hardly observed during the day light period. Hence, the only solution to study these species of wildlife is the application of camera-trapping systems. In Deng-Deng national park the presence of pangolin species was identified through the camera-trapping system. This discovery has created more understanding on the species presence and status in this protected area. The population of pangolins has much been pressured by hunting for its scale-market in some African and Asian countries. The ancient tradition of pangolin poaching for only household bushmeat consumption is met with high pangolin scale demand for traditional medicine, the main reason for its scarcity in the wild and in the village-market areas where it was commonly sold by the local hunters. This study is presumably a baseline for future pangolin ecological surveys within the national park.
References
- Abernethy, K.A., Coad, L.M., Taylor, G., et al. Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. (2013) Philosophical Transactions of the royal Society B
- Acrenaz, M.A.J., Hearn, J., Ross, R., et al. Handbook for Wildlife Monitoring using Camera Traps. (2012) BBEC II Secretariat, Kota Kinabalu, Sabah, Malaysia, 71pp.
PubMed│CrossRef│Others
- Andama, E. Checklist of the mammals of Bwindi Impenetrable National Park, South-Western Uganda. (2000a) Unpublished report. Institute of Tropical Forest Conservation, Bwindi.
PubMed│CrossRef│Others
- Akpona, H.A., Djagoun, C.A.M.S., Sinsin, B. Ecology and ethnozoology of the three-cusped pangolin Manistricuspis (Mammalia, Pholidota) in the Lama Forest Reserve, Benin. (2008) Mammalia 72:198-202.
PubMed│CrossRef│Others
- Azlan, J.M., Sharma, D.S.K. The diversity and activity patterns of wild felids in a secondary forest in Peninsular Malaysia. (2006) Oryx 40(1): 36–41.
- Baillie, J., Challender, D., Kaspal, P., et al. Manis crassicaudata. The IUCN Red List of Threatened Species. (2015) Version.
PubMed│CrossRef│Others
- Bruce, T., Wacher, T., Ndinga, H., et al. Camera-trap survey for larger terrestrial wildlife in the Dja Biosphere Reserve, Cameroon, Diversity & Intactness of the Larger Vertebrate Fauna. (2017) Cameroon: Zoological Society of London (ZSL) & Cameroon Ministry of Forests and Wildlife (MINFOF).
PubMed│CrossRef│Others
- Chakkaravarthy, Q.A. Research and conservation needs of the Indian pangolin (Manis crassicaudata). (2012) Proceedings of Third Seminar on Small Mammals Conservation Issues 50-55pp.
PubMed│CrossRef│Others
- Challender, D. Asian pangolins: Increasing affluence driving hunting pressure. (2011) TRAFFIC Bulletin 23(3): 92-93.
PubMed│CrossRef│Others
- Challender, D., Hywood, L. African pangolins: under increased pressure from poaching and intercontinental trade. (2012). TRAFFIC Bulletin 24(2): 53-55.
PubMed│CrossRef│Others
- Cheng, W., Zing, S., Bonebrake, T.C. Recent pangolin seizures in China reveal priority areas for intervention. (2016) Conserv Lett 10(6): 757-764
- Cutler, T.L., Swann, D.E. Using remote photography in wildlife ecology: a review. (1999) Wild Soc Bull 27(3): 571-581
PubMed│CrossRef│Others
- Gaubert, P. Family manidae (Pangolins). In: Wilson D.E $Mittermeier R. A (EDS). Hand book of the mammals of the world: vol. 2: Hoofed mammals. (2011) Lynx Edicions 82-103
PubMed│CrossRef│Others
- Giman, B., Stuebing, R., Megum, N., et al. A camera trapping inventory for mammals in a mixed use planted forest in Sarawak. (2007) Raffles Bull Zool 55(1): 209-215.
PubMed│CrossRef│Others
- Heath, M.E., Coulson, I.M. Preliminary studies on relocation of Cape pangolins (Manis temminckii) South Afr J of Wil Res 27(2): 51-56.
PubMed│CrossRef│Others
- Kasangaki, A., Kityo, R., Kerbis, J. Diversity of rodents and shrews along an elevational gradient in Bwindi Impenetrable National Park, south-western Uganda. (2003) Afr J Ecol 41(2): 115-123.
- Kaspal, P. Status, distribution, habitat utilization and conservation of Chinese Pangolin in the community forests of Suryabinayak. (2008)
PubMed│CrossRef│Others
- Kelly, M.J., Noss, A.J., Di Bitetti, M.S., et al. Estimating Puma densities from camera trapping across three study sites: Bolivia, Argentina, and Belize. (2008) J Mammal 89(2): 408-418.
- PubMed│CrossRef│Others
- Kingdon, J. The Kingdom Field Guide to African Mammals (2nd edition), London, Bloomsbury Publishing Plc. (2015).
PubMed│CrossRef│Others
- Lim, N.T., Ng, P.K. Home range, activity cycle and natal den usage of a female Sunda pangolin (Manis javanica) (Mammalia: Pholidota) in Singapore. (2008) Endang Spec Res 4: 233-240.
- Lim, N.T., Ng, P.K. Home range, activity cycle and natal den usage of a female Sunda pangolin (Manis javanica), (Mammalia: Pholidota) in Singapore. (2007) Endang Spec Res.
PubMed│CrossRef│Others
- Maffei, L., Noss, A.J. How Small is too Small? Camera trap survey areas and density estimates for Ocelots in the Bolivian Chaco. (2008) Biotropica 40(1): 71-75.
- Maisels, F., Ambahe, E., Ambassa, B., et al. Wildlife and human impact surveys of the Deng Deng National Park and UFA 10065, 2010. WCS Cameroon, Yaounde, Cameroon. (2010).
PubMed│CrossRef│Others
- Marler, P.N. Camera trapping the Palawan Pangolin (Manis culionensis) (Mammalia: Pholidota: Manidae) in the wild. (2016) J Threat Taxa 8(12): 9443-9448.
- Norman, T. Ecological research and conservation of Sunda Pangolin (Manis javanica) in Singapore. (2009) Workshop on trade and conservation of pangolins native to south and Southeast Asia. Citeseer 90.
PubMed│CrossRef│Others
- O’brien, T.G., Kinnaird, M.F., Wibisono, T.H. Estimation of Species Richness of Large Vertebrates Using Camera Traps: An Example from an Indonesian Rainforest. Camera Traps in Animal Ecology. (2011)
PubMed│CrossRef│Others
- Pabasara, G. Assessment of the abundance and habitat preference of Indian pangolin (Manis crassicaudata) in yagirala forest reserve; a tropical lowland forest in south-west Sri Lanka. (2016)
PubMed│CrossRef│Others
- Plumptre, A.J. Monitoring mammal populations with line transect techniques in African forests. (2000) J Anim Ecol 37(2): 356-368.
- Plumptre, A.J., Reynolds, V. Nesting behavior of Chimpanzees: Implications for Censuses. (1997) Int. J. Primatol 18(4): 475-485.
- Richer, R., Coulson, I., Heath, M. Foraging behaviour and ecology of the cape pangolin (Manis temminckii) in north‐western Zimbabwe. (1997) Afr J Ecol 35(4): 361-369.
- Rovero, F., Collett, L., Ricci, S., et al. Distribution, occupancy, and habitat associations of the gray-faced sengi (Rhynchocyon udzungwensis) as revealed by camera traps. (2013) J Mamm 94(4): 792-800.
- Shreya, D. ‘Forgotten forests’ of South Sudan: Camera traps capture first-ever pictures of forest elephants, giant pangolins in the country. (2015)
PubMed│CrossRef│Others
- Silveira, L., Ja′como, A.T.A., Filho, A.J.F.D. Camera trap, line transect census and track surveys: a comparative evaluation. (2003) Biol Conserv 114(3): 351-355.
- Suwal, T.L. Status, Distribution, Behaviour and Conservation of Pangolins in private and Community Forest of Balthali in Kavre, Nepal. (2011)
PubMed│CrossRef│Others
- Tobler, M.W., Carrillo-Percastegui, S.E., Pitman, R.L., et al. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. (2008) Anim Conserv 11(3): 169-178.
- Treves, A., Mwima, P., Plumptre, A., et al. Camera-trapping forest–woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. (2010) Biol Conser 143(2): 521-528.
- Whitworth, A., Braunholtz, L.D., Huarcaya, R.P., et al. Out on a limb: Arboreal camera traps as an emerging methodology for inventorying elusive rainforest mammals. (2016) Trop Conser Sci 9(2): 675-698.
- Wilcox, D., Hao, D.T., Phuong, T.Q. An internal report on the collaborative survey between the Carnivore and Pangolin Conservation Program and the Ngoc Son Ngo Luong Project carried out between February - March. (2011)
PubMed│CrossRef│Others
- Wilson, G.J., Delahay, R.J. A review of methods to estimate the abundance of terrestrial carnivores using field signs and observation. (2001) Wild Res 28(2): 151-164.
- Yasuda, M. Monitoring diversity and abundance of mammals with camera traps: a case study on Mount Tsukuba, central Japan. (2004) Mammal Study 29(1): 37-46.
- Zoological Society of London, Congo Basin Institute, & MINFOF. (2017). Giant pangolin video from Bouamir Research Station, Dja Biosphere Reserve, Cameroon.
PubMed│CrossRef│Others












