AURASTOP® in the Treatment of Migraine Aura
F. Antonaci1,3, V. Rebecchi4, G. Sances1, P. Merlo5, A. Giorgetti6, F. Di Palma7, E. Matta8, C. Dallocchio9, C. Tassorelli1,3, A.Pezzini10, G. Dalla Volta11*
Affiliation
1 Headache Science Centre, Istituto Neurologico Nazionale Mondino, Pavia
2 UC Neurologia Speciale d’Urgenza, Istituto Neurologico Nazionale, Pavia
3 Dipartimento di Scienze del Sistema Nervoso e del Comportamento Università di Pavia
4 Centro Cefalee UOC Neurologia -Varese- ASST Settelaghi – Università dell’ Insubria
5 U.O.Neurologia- Centro Cefalee, Humanitas Gavazzeni, Bergamo
6 Centro Cefalee, Dipartimento di Neuroscienze H di Legnano ASST Ovest milanese
7 Centro Cefalee UOC Neurologia della ASST Lariana-Ospedale S. Anna di Como
8 Centro Cefalee UOC neurologia ASST Bergamo ovest
9 U O Neurologia, ASST Pavia, Ospedale Civile, Voghera
10 Dipartimento di Scienze Cliniche e Sperimentali, Clinica Neurologica, Università degli Studi di Brescia
11 Centro Cefalee U.O Neurologia - Istituto Clinico Citta’ di Brescia, Brescia
Corresponding Author
Giorgio Dalla Volta, Centro Cefalee U.O Neurologia- Istituto Clinico Città Di Brescia,Via Gualla, 25123 Brescia, Italy, E-mail: dalla@numerica.it; ncszn1234@gmail.com
Citation
Dalla Volta, G., et al. AURASTOP® in the Treatment of Migraine Aura. (2018) Int J Neuro Brain Dis 5(1): 11- 14
Copy rights
© 2018 Dalla Volta, G. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Migraine aura; Tanacetum Partenium; Headache; Treatment
Introduction
The phenomenon of aura is traditionally considered as a non-avoidable event occurring in about one quarter of migraine sufferers before the headache phase[1]. Although previous trials on migraine treatment have clearly demonstrated the benefit conferred by some agents, including triptans, on migraine headache, as well as their time-dependent effect, the therapeutic potential of modulating the phenomenon of migraine aura before pain occurrence has not been investigated so far. At least theoretically, interventions aimed at limiting migraine aura might also have an influence on pain control. Aura symptoms frequently persist beyond the 1-hour limit set by the International Headache Classification diagnostic criteria[2], with many accompanying manifestations (prostration, confusion, difficulty concentrating among the others) lasting up to 24 hours and whose complete resolution represents the end of the crisis. During this phase the patient experiences a high degree of disability by being forced to interrupt many activities of daily life as well as of psychological stress, though the symptoms of aura tend to recur with the same features over time.
To date, sparse studies have attempted to investigate the efficacy of selected molecules on the phenomenon of aura. Among these, the intranasal administration of ketamine proved effective in reducing the severity but not the duration of the aura[3]. Similarly, tonabersat as well as the intra-venous administration of prochlorperazine were tested in small studies or in anecdotal reports, but they did not receive approval for clinical use[4,5].
Over the last years, numerous nutraceutical compounds have been proposed for the preventive treatment of migraine with or without aura, leading many scientific societies to report ad hoc guidelines to support practitioners in their use[6]. In particular, the feverfew (Tanacetum Partenium) has been used for many years in the UK[7], and 5-hydroxy tryptophan for the prevention of migraine in Italy.
Based on what above, in the present study we tested the hypothesis that an association of molecules that has been proven to drastically reduce the duration and degree of disability of the aura symptoms can also influence the severity of pain that follows the aura itself. In particular, we investigated the therapeutic potentials of a new compound (Aurastop®), derived from the combination of tanacetum partenium (150 mg extracted at 0,8 % = 1,2 mg di of active partenolide), griffonia simpliciofila (20 mg of 5-HTP) and magnesium (185 mg of magnesium pidolatum).
Patients and Methods
The present study is supported by the Società Italiana per lo Studio delle Cefalee (SISC), Lombardia section.
Participants
Patients with personal history of headache fulfilling ICHD-3 beta criteria for the diagnosis of migraine with aura were recruited at the Headache Centre of each participating hospitals.
Diagnosis of headache was made by experienced headache specialists, and each patient underwent a detailed clinical and neurological examination.
The following inclusion criteria were considered:
1) age between 18 and 60 years
2) diagnosis of migraine with aura, characterized by at least 3 episodes of aura/year with a minimum aura duration of 20 minutes.
The introduction of a preventive treatment during the observation period was considered an exclusion criterion. This study was an audit of outcome and, as such, according to the Italian guidelines, did not require ethics committee approval.
All participants received the combination of tanacetum parteninum (150 mg extracted at 0.8% = 1.2 mg of active parthenolide), griffonia simpliciofila (100 mg of 5-HTP) and magnesium (185 mg of magnesio pidolate) (Aurastop®).
Study design
At baseline (t0), the natural history of aura phenomenology was studied. To this purpose, each patient received a migraine headache diary, to keep track of aura and headache characteristics of the following 3 episodes. In particular, aura subtype (only visual, visual and somatosensory, visual, somatosensory and speech/language symptoms [here defined as complex]) aura duration, disability (on a scale ranging from 0 to 5), presence of concomitant/following headache characteristics (duration, intensity [Visual Analogic Scale, 0 to 10]), use of common pain medications (triptans, nonsteroidal anti-inflammatory drugs), and response to symptomatic treatment were considered.
After 3 episodes of aura (with or without migraine) migraine headache diary of each patient were re-evaluated (t1) considering inclusion/exclusion criteria and aura characteristics. Then, each patient received a blister with 6 tablets of Aurastop®, with the instruction to assume a tablet of Aurastop® at the beginning of the following 3 auras, recording aura characteristics on migraine headache diary as usual and a second tablet at the beginning of the pain (in case pain occurred). Patients were allowed to take the usual pain killer after 1 hour in case of persistent pain. Each patient and migraine headache diary data were further evaluated (t2) after these 3 aura episodes.
The primary end-point of the study was a reduction > 50% of duration and disability of the aura phenomena. The secondary end-point was the modification of the headache features after the aura (in particular, duration, intensity, assumption of usual analgesic-triptans, and efficacy of the pain killer [on a 0 to 5 scale, with 0 indicating no pain and 5 indicating maximum pain severity]).
Statistical Analysis
Dependent variables were compared by McNemar’s χ² analysis. Wilcoxon’s signed rank test was used to compare migraine aura and headache characteristics before and after treatment. Statistical analyses were performed using SPSS 21.0 (IBM SPSS Statistics 2013, Armonk, NY, USA).
Results
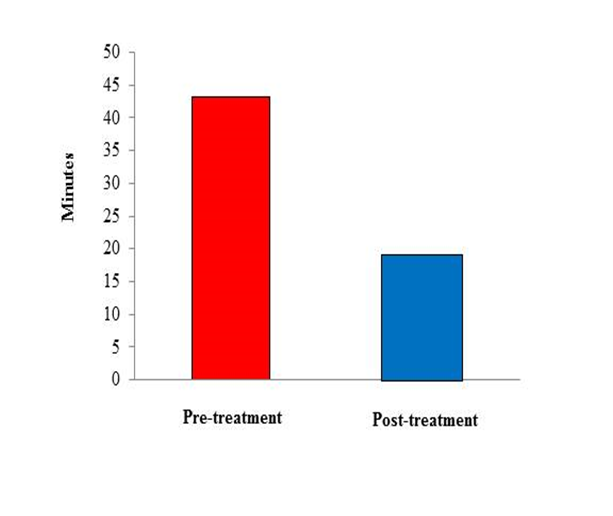
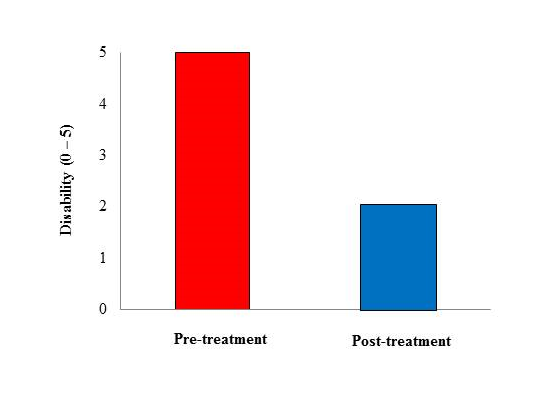
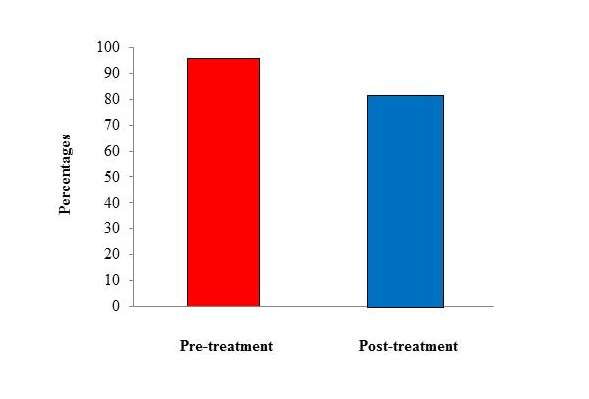
Two-hundred subjects with a diagnosis of migraine with aura (ICHD-3 beta criteria) qualified for the present study (mean age 33 ± 1,5 years [range, 18 – 54], males, 83 [33.2%]). As summarized in Table 1, Aurastop® determined a reduction of the duration of aura (t1= 43.2 ± 19.3 minutes vs t2 = 18.2 ± 10.3 minutes, p < 0.01), as well as of the degree of disability (t1 = 5 [4-5] vs t2 = 2 [1-2], p < 0.01). In particular, a 4-5 degree of disability was observed in more than 90% of patients before treatment versus a 1-2 degree in more than 90% of patients after treatment. Furthermore, the characteristics of migraine aura also changed after treatment with Aurastop®, with an obvious reduction of its complexity (p > 0.01), as well as of somatosensory manifestations (111 ± 18.5 before treatment vs 20 ± 3.3 after treatment). We also detected a significant reduction of headache crises (p < 0.01), of pain severity and duration (p < 0.01), of the number of analgesics assumed by each patient, and an increased level of efficacy determined by the same analgesics or triptans after treatment with Aurastop®.
Table 1: Primary and secondary endpoints
| Migraine characteristics | Pre-treatment | Post-treatment | p-value |
| Migraine aura | |||
| Visual, n (%) | 600 (100) | 594 (99.0) | |
| Sensory, n (%) | 111 (18.5) | 20 (3.3) | ≤ 0.001 |
| Complex, n (%) | 29 (4.8) | 1 (0.2) | ≤ 0.001 |
| Duration, minutes, mean ± SD | 43.2±19.3 | 182 ± 10.3 | ≤ 0.001 |
| Disability (0-5), median (IQR) | 5 (4-5) | 2 (1-2) | ≤ 0.001 |
| Headache, n (%) | 577 (96.2) | 451 (16.7) | ≤ 0.001 |
| Duration, hoU’s, mean ± SD | 48.0 ± 13.3 | 3.8 ± 3.7 | ≤ 0.001 |
| Pain intensity (O-10) | 5 (4 -7) | 2 (1-2) | ≤ 0.001 |
| Beneit (0-5) | 2 (1-2) | 5 (4-5) | ≤ 0.001 |
| Useofanalgesics, n (%) | 576 (96.0) | 372 (19.8) | ≤ 0.001 |
Figure 1: Histogram of aura duration pre- and post-treatment with Aurastop®
Figure 2: Histogram of aura-related disability pre- and post-treatment with Aurastop®.
Figure 3: Histogram of use of analgesics pre- and post-treatment with Aurastop®.
Our data also provide evidence that the eventual assumption of a second tablet of Aurastop® at the beginning of the pain, according to the pre-specified protocol, did not substantially modify pain severity, duration of the headache phase, or the individual sensitivity to analgesics. No major side effects or worsening of migraine characteristics were detected and associated with the use of Aurastop®.
Discussion
The main finding of the present study is the observation of a ~ 96% reduction of self-reported aura episodes in patients who shifted from a standard treatment approach to the regimen including the new compound Aurastop®. Such a reduction concerned not only the duration of aura, but also the degree of disability determined by this phenomenon, which provides support to the hypothesis that Aurastop® may modulate cortical neuronal hyperexcitability and, consequently, modify the clinical features of aura itself. Furthermore, the observation that aura was no more followed by a headache phase in about 30% of patients, that pain severity and duration were significantly reduced, and that the efficacy of analgesics/triptans was increased in patients assuming Aurastop® should be regarded as indirect demonstration that the new compound may interfere with the peripheral-central sensitization and with the TRPA1- and NMDA-dependent synaptic transmission as well.
A number of biologic effects may explain the clinical benefit conferred by Aurastop® in the treatment of migraine aura. First, Mg+, a principal component of the new agent, has a well-known inhibitory effect on NMDA receptor activity which is likely to contrast the CSD phenomenon[8,9]. Second, 5-HTP influences the kynurenine pathway, leading to an increase of kyna levels which, in turn, further inhibits the activity of NMDA receptors and the activation of the trigemino-vascular system, and also rapidly (kyna passes the blood-brain barrier [BBB]) interferes with CSD and BBB integrity[10-12]. Finally, partnolide, the active metabolite of tanacetum partenium, is an active agonist of TRPA1 receptors implicated in the release of CGRP from the perivascular terminals of neurons involved in neurogenic inflammation, the key mechanism leading to head pain[13]. The short delay between oral assumption of Aurastop® and the clinical effect on aura symptoms is well explained by the pharmacokinetic of tanacetum partenium, which, according to several results obtained in animal models (citare), is characterized by a very short time for elution (1.3 minutes), absorption (~3 minutes), and passage through the BBB[14].
The results of this new analysis are well in line with those previously derived by the same Authors from a smaller independent cohort[15] and emphasize the therapeutic potentials of Aurastop®, though they were obtained in the setting of an observational study without a placebo group. Many molecules, however, have been widely used in medicine for years based only on clinical evidence of efficiency (i.e, beta-blockers for migraine prevention long before the pathogenesis of migraine was elucidated). On the other side, data from animal models provide strong support to the biologic effect of Aurastop®, in particular of one of its components, the tanacetum parthenium, in modulating the cortical electric phenomenon underlying migraine aura[11].
Conclusion
In conclusion, the results of the present study strengthen the sparse anecdotal observations that Aurastop® may influence the early phases of migraine aura and cortical spreading depolarization resulting in pain and the accompanying symptoms. From a biological perspective, they might be regarded as a step forward in better understanding the basic mechanisms of the cortical hyperexcitability leading to headache.
References
- 1. Charles, A., Hansen, J.M. Migraine Aura: New Ideas about Cause, Classification, and Clinical Significance. (2015) Curr Opin Neurol 28(3): 255-260.
- 2. The International Classification of Headache Disorders. 3rd Edition (Beta Version).
PubMed||CrossRef||Others
- 3. Afridi, S.K., Giffin, N.J., Kaube, H., et al. A randomized controlled trial of intranasal ketamine in migraine with prolonged aura. (2013) Neurology 80(7): 642-647.
- 4. Hauge AW, et al. Effects of tonabersat on migraine with aura: a randomised, double-blind, placebo-controlled crossover study. (2009) Lancet Neurol 8(8): 718-723.
- 5. Rozen, T.D. Aborting a prolonged migrainous aura with intravenous prochlorperazine and magnesium sulfate. (2003) Headache 43(8): 901-903.
- 6. Rajapakse, T., Pringsheim, T. Nutraceuticals in Migraine: A Summary of Existing Guidelines for Use. (2016) Headache 56(4): 808-816.
- 7. Diener, H.C., Pfaffenrath, V., Schnitker, J., et al. Efficacy and safety of 6.25 mg t.i.d. feverfew CO2-extract (MIG-99) in migraine prevention--a randomized, double-blind, multicentre, placebo-controlled study. (2005) Cephalalgia 25(11): 1031-1041.
- 8. Petrusic, I., Zidverc-Trajkovic, J. Cortical spreading depression: origins and paths as inferred from the sequence of events during migraine aura. (2014) Funct Neurol 29(3): 207-212.
- 9. Sun-Edelstein, C., Mauskop, A. Role of magnesium in the pathogenesis and treatment of migraine. (2009) Expert Rev Neurother 9(3): 369-379.
- 10. Csáti, A., Edvinsson, L., Vécsei, L., et al. Kynurenic acid modulates experimentally induced inflammation in the trigeminal ganglion. (2015) J Headache Pain 16: 99.
PubMed||CrossRef||Others
- 11. Chauvel, V., Vamos, E., Pardutz, A., et al. Effect of systemic kynurenine on cortical spreading depression and its modulation by sex hormones in rat. (2012) Exp Neurol 236(2): 207-214.
- 12. Olàh, G., Herédi, J., Menyhàrt, A., et al. Unexpected effects of peripherally administered kynurenic acid on cortical spreading depression and related blood–brain barrier permeability. (2013) Drug Des Devel Ther 16: 981–987.
- 13. Tassorelli, C., Greco, R., Morazzoni, P., et al. Parthenolide is the component of Tanacetum parthenium that inhibits nitroglycerin-induced Fos activation: studies in an animal model of migraine. (2005) Cephalalgia 25(8): 612-621.
- 14. Zhao, A.Q., Zhao, J.H., Zhang, S.Q., et al. Determination of parthenolide in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. (2016) J Pharm Biomed Anal 119: 99-103.
- 15. Zavarise, P., Dalla Volta, G. A Combination of Tanacetum parthenium, Griffonia simplicifolia and Magnesium (Aurastop®) as Symptomatic Acute Treatment for Migraine Aura: A Retrospective Cohort Study. (2017) Open Access Library Journal 4(6): 1-9.