An Overview of the Experimental Islet Isolation In Human Pancreas
Kursun, A.O1,2 Kasapoglu, P1,2, Aydin B1,2, Adas CU1,2, Ozdemir A1,2, Cevik A4, Kucuk M4, Cakiris A5, Kuçuksezer UC2, Ustek D5, Suzergoz F1,2,6,Bakkaloglu H7, Aydin AE7, Deniz G2, Karsidag K8, Yilmaz MT1,2,8
Affiliation
- 1Diabetes Application and Research Center (DIYAM), Istanbul University, Sehremini, Istanbul
- 2Department of Immunology, Institute of Experimental Medicine (DETAE), Istanbul University, Sehremini, Istanbul
- 3Department of Medical Pharmacology, Istanbul Medicine Faculty, Istanbul University, Capa, Istanbul
- 4Department of Laboratory Animals Science, DETAE, Istanbul University, Sehremini , Istanbul
- 5Department of Genetics, DETAE, Istanbul University, Sehremini , Istanbul
- 6Department of Biology, Faculty of Arts and Sciences, Harran University, Sanliurfa
- 7Department of General Surgery, Transplantation Unit, Istanbul Medicine Faculty, Istanbul University, Capa, Istanbul
- 8Department of Internal Medicine, Endocrinology and Metabolism Diseases Division, Istanbul Medicine Faculty, Istanbul University, Capa, Istanbul- Turkey
Corresponding Author
Ali Osman Gurol, Associate Professor, Diabetes Application and Research Center (DIYAM), DETAE Building, Istanbul University, 34393, Sehremini / Istanbul- Turkey. Tel: +90 544 794 3144; E-mail: ogurol@istanbul.edu.tr
Citation
Gurol, A.O., et al. An Overview of the Experimental Islet Isolation in Human Pancreas.(2015) Cell Immunol Serum Biol 1(1): 1- 3.
Copy rights
©2015 Gurol, A.O. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Keywords
Type 1 diabetes; Islet isolation; Human; Auto-transplantation
Abstract
Type 1 diabetes mellitus (T1DM) is a disease caused by autoimmune destruction of the pancreatic beta cells. Allogeneic islet transplantation is recommended for “brittle T1DM patients “ as intensive insulin therapy seems to be associated with an increased incidence of hypoglycemia. The aim of this article was to describe the studies on human islet isolations verified in the Diabetes Application and Research Center (DIYAM) in Istanbul University. Pancreas were harvested from brain-dead donors and stored at -80°C. Media preparation [dilution, priming, wash, final wash, decontamination and gradient purification solutions, University of Wisconsin (UW) pre-purification media]; instrument set-up (dissection/perfusion and dissociation systems); distension [pancreas preparation (trimming and rinsing of the pancreas, cannulation into the pancreatic duct)]; making enzyme solution (reconstitution of enzyme, dilution of enzyme solution, filtration of diluted enzyme solution); perfusion (perfusion to distend pancreas with enzyme solution); dissociation (pancreas digestion, digested tissue collection); recombination and purification (using COBE 2991cell processor) were the main phases of islet isolations. As the primary goal of isolating the pancreatic islets for either in vivo transplantation or in vitro studies is to achieve a large amount of viable islets, also viability experiments both in vitro and in vivo using experimental animals were realized in these studies. An average of 300,000 islets were obtained after purification. We suggest that improvement in amount and viability of isolated islets after long-term storage can be achieved when appropriate storage solutions and new types of enzymes would be used, and in the near future DIYAM would be among islet transplantation centers worldwide by starting auto-transplantations of islets of Langerhans for mantaining insulin production and secretion in pancreotectomized patients.
Introduction
Diabetes is a disease of vascular system which can lead to rapid atherosclerosis[1,2]. For this reason, diabetes is a multi-system disease with capacity to lead disorders of almost all systems including cardiovascular, renal, ophthalmologic and neurologic systems, and a main disposing factor to neurologic diseases and coronary artery disease[3]. Half of hemodialysis patients are diabetic. Diabetes is the main reason of blindness in people over 20 years of age[4]. For all of the mentioned reasons, diabetes is a major health problem of societies, which draws interest of all disciplines of medicine.
Type 1 diabetes mellitus (T1DM) is a disease caused by autoimmune destruction of the pancreatic beta cells, and due to the consequent shortage of insulin, patients need exogenous insulin for survival[5,6]. However, despite the use of the intensive insulin treatment is executed, chronic complications of the disease are inevitable and unfortunately associated with high morbidity and mortality[7]. The replacement of beta cells by whole pancreas or islet transplantation is the only way to restore the physiological secretion of insulin. Although total pancreas transplant recoveries adequate glycemic control and is able to prevent diabetic complications[8], this procedure is usually recommended in patients with T1DM who need a kidney transplant or already transplanted and are under an immunosuppressive regimen. From this point of view, also due to high morbidity and mortality associated with surgery in pancreas transplantation, allogeneic islet transplantation which is much less risky is recommended for “brittle T1DM patients”[7,9,10]. It’s made by a simple method of cell infusion into the intrahepatic, and recently also into omental environment, and does not lead to serious hypoglycemic episodes that are associated with intensive insulin therapy[11].
For this very important reason, Diabetes Application and Research Center in Istanbul University was established, and aims to develop and apply research projects, converting molecular genetical research with the aim of limiting the organ failures related with diabetes.
The basic component of the Center has a research lab (a Clean Room) with all required infrastructure. Being the first in Turkey to isolate islets of Langerhans from human pancreata, and as experiments of each center participating in clinical research in islet transplantation is based on information obtained from studies conducted using animal models[12,13], our center is also pioneering experimental isolation and transplantation of islets of Langerhans and helding educational courses on experimental animals such as rats and pigs since 1990.
Human Islet Isolation in DIYAM
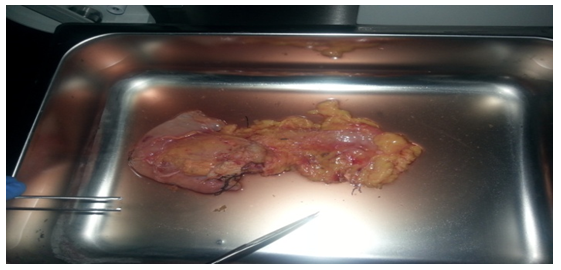
We obtained the pancreata (Figure 1) from brain-dead donors and isolated islets according to “Current Human Islet Isolation Protocol”[14] edited by Lakey JRT et al.; briefly, to maintain cold chain kept the pancreata firstly in 0.9% isotonic sodium chloride or University of Wisconsin Solution (UW) for about 30 to 60 minutes until arrival to the clean room; then stored at -80°C. When working, depending on the weight of organs, beakers containing one percent of Liberase HI (Liberase; Boeringer-Mannheim/Roche) were placed on ice. Then the enzymes were kept at room temperature for about 5 minutes, later diluted with cold Hank’s balanced salt solution (HBSS). The digestive system consisting of silicone tubes, Ricordi Chamber, peristaltic pump, water bath were reached at 37°C. Pancreata were perfused on perfusion unit by infusion of Liberase HI/HBSS mix. The tissues were pieced and placed into Ricordi Chamber. Remaining Liberase HI/HBSS mix added to the system, then circulation started by adding dilution solution. The collection phases continued with recombination and purification of islets by using COBE 2991 cell processor from acinar tissue[14]. Acinar cells started to be seen as blocks in samples that were taken at 10 minutes. Separated acinar cells were detected in 16 minutes. Acinar cells increased from 17 minutes and free islets began to appear. And then, number of free islets began to increase (Figure 2). During the islet recombination phase, fluorescein diacetate and propidium iodide (FDA/PI) stain was used to assess the viability of the islets. In addition, samples were stained with dithizone (DTZ) for detecting islets. In a second step, after staining with trypan blue some of cells appearing alive under light microscopy, captured the added DTZ, while others did not. Thus we thought that a part of living cells were islets. To detect the viability in vivo, nearly 1500 islets were transplanted intraportally to inbred Wistar albino rats which were made diabetic by streptozotocin (STZ) and fed with regular rodent chow whose glycemia values (mg/dl) were obtained from vein blood samples on days –1, 0, +1, +2, and +195. Slightly lower levels were found on early post-transplant days +1 vs –1 (p = 0.045) and 0 (p = 0.028). An increase was detected on day +2 vs +1 (p = 0.077). No statistical difference was noted on day +195. An average of 300,000 islets with diameter 50-350 mm but mostly 50-100 mm were obtained after purification.
Figure 1: Whole pancreas obtained from brain-dead donor. It was processed for isolating Islets of Langerhans.
Figure 2: Human islets isolated during the recombination phase of islet isolation. Figure shows direct image of human islets of Langerhans under dissecting microscope. In the isolation process, first, acinar cells were seen as blocks. Following the increase of the number of acinar cells, free islets began to appear and increase.
Conclusion
The donor factors and variables in the isolation process can affect the ability to obtain successful human islet isolation[15]. Our results suggest that by conserving pancreatic tissue for more than one month at -80°C, it’s still possible to obtain viable islets, even though it would be less in number. We think that viable islet yield may increase when appropriate storage solutions[16] and new types of enzymes could be used. Furthermore, it seems that at least for auto-transplantation, it could be possible to isolate viable islets at a sufficient amount, remote to operation of pancreatomized patients who cannot be auto-transplanted on the same day due to various factors. However, further investigations should be performed for the realization of our thoughts. Until today, our Centre conducted 25 human islet isolations (14 isolations from February 2014 by the license renewal of the Ministry of Health) and is able to verify auto-transplantation of islets of Langerhans as soon as the legal proceedings are completed.
References
- 1. Russell, M., Fleg, J.L., Galloway, W.J., et al. Examination of lower targets for low-density lipoprotein cholesterol and blood pressure in diabetes--the Stop Atherosclerosis in Native Diabetics Study (SANDS). (2006) Am Heart J 152(5): 867-875.
- 2. Bornfeldt, K.E. Uncomplicating the Macrovascular Complications of Diabetes: The 2014 Edwin Bierman Award Lecture. (2015) Diabetes 64(8): 2689-2697.
- 3. Diagnosis and classification of Diabetes Mellitus. (2013) Diabetes Care 36(Suppl 1): S67-S74.
- 4. Infeld, D.A., O’Shea, J.G. Diabetic retinopathy. (1998) Postgrad Med J 74(869): 129–133.
- 5. Kobayashi, N. The current status of islet transplantation and its perspectives. (2008) Rev Diabet Stud 5(3): 136-143.
- 6. Pugliese, A., Skyler, J.S. George S. Eisenbarth: insulin and type 1diabetes. (2013) Diabetes Care 36(6): 1437-1442.
- 7. Rheinheimer, J., Bauer, A.C., Silveiro, S.P., et al. Human pancreatic islet transplantation: an update and description of the establishment of a pancreatic islet isolation laboratory. (2015) Arch Endocrinol Metab 59(2): 161-170.
- 8. Vardanyan, M., Parkin, E., Gruessner, C., et al. Pancreas vs. islet transplantation: a call on the future. (2010) Curr Opin Organ Transplant 15(1): 124-130.
- 9. Gruessner, R.W., Sutherland, D.E., Kandaswamy, R., et al. Over 500 solitary pancreas transplants in nonuremic patients with brittle diabetes mellitus. (2008) Transplantation 85(1): 42-47.
- 10. Ichii, H., Ricordi, C. Current status of islet cell transplantation. (2009) J Hepatobiliary Pancreat Surg 16(2): 101-112.
- 11. Leitao, C.B., Tharavanij, T., Cure, P., et al. Restoration of hypoglycemia awareness after islet transplantation. (2008) Diabetes Care 31(11): 2113-2115.
- 12. Hogan, A., Pileggi, A., Ricordi, C. Transplantation: current developments and future directions; the future of clinical islet transplantation as a cure for diabetes. (2008) Front Biosci 13: 1192-1205.
- 13. Carter, J.D., Dula, S.B., Corbin, K.L., et al. A Practical Guide to Rodent Islet Isolation and Assessment. (2009) Biol Proced Online 11: 3-31.
- 14. Lakey, J.R.T., Kobayashi, N., Shapiro, A.M.J., et al. Current Human Islet Isolation. (2004) Medical Review Co., Ltd. Osaka, Japan
- 15. Kim, S.C., Han, D.J., Kang, C.H., et al. Analysis on donor and isolation-related factors of successful isolation of human islet of Langerhans from human cadaveric donors. (2005) Transplant Proc 37(8): 3402-3403.
- 16. Shimoda, M., Itoh, T., Sugimoto, K., et al. An effective method to release human islets from surrounding acinar cells with agitation in high osmolality solution. (2011) Transplant Proc 43(9): 3161-3166.