Anti-NMDA receptor encephalitis associated to ovarian teratoma: physiopathology and when to suspect
Letícia Caroline Breis*, Marco Antônio Machado Schlindwein, Marcus Vinicius Magno Gonçalves
Affiliation
Department of Medicine, University of the Region of Joinville, Brazil
Corresponding Author
Letícia Caroline Breis, Univertisy of the Region of Joinville, Rua Ministro Calógeras, 439, Bucarein, Joinville, Santa Catarina, Brazil, 89202-207, Tel: +5547991760101; E-mail: breisleticia@gmail.com
Citation
Breis, L.C., et al. Anti-NMDA Receptor Encephalitis Associated with Ovarian Teratoma: Pathophysiology and When to Suspect. (2020) Int J Neurol Brain Dis 7(1): 7-10.
Copy rights
© 2020 Breis, L.C. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Ovarian teratoma; Anti-NMDA; Encephalitis
Abstract
Antibodies against the glutamate receptors NMDA can cause autoimmune encephalitis. This condition occurs especially in young women and the prevalence of associated ovarian have been described as almost 20%. Patients present with new onset neuropsychiatric symptoms and tend to show no improvement with antipsychotic medication, increasing suspicion of an underlying cause. When searching for ovarian teratoma, the absence of a detectable tumor on image exams do not exclude this hypothesis, once tumors may be too small to de detected in image exams or may also develop after the neoplastic syndrome, and therefore these patients must be followed-up. It is important to recognize the epidemiology and to be aware of when to suspect about this disease, once early treatment (surgical resection and immunotherapy) is associated with greater prognosis.
Introduction
Anti-NMDA Receptor Encephalitis
Anti-NMDA receptor (anti-NMDAR) encephalitis is an autoimmune encephalitis that occurs especially in young women and has a high association with tumors. Almost 80% of the patients are female[1], and ovarian teratoma is the most associated cause[1-4].
Among encephalitis, infectious diseases are the most frequent and reminded causes (especially Herpes simplex virus). However, it is important to recognize other causes, such as vasculitis, malignancy and, as for anti-NMDAR encephalitis, paraneoplastic syndromes[1]. Anti-NMDAR encephalitis may also occur in the absence of a neoplasm, which means, as a primary disease, but it represents a minority of cases[5].
Although men and children can be affected by anti-NMDAR encephalitis[5], the most common presentation is a female patient (median age: 23 years)[5] with psychiatric symptoms (behavioral changes, confusion, memory deficits, psychosis and hallucination)[6,7] and seizures, and therefore psychiatrists are often the first professionals to see the patient[7,8]. Also, patients tend to show no improvement with antipsychotic medications and rapidly evolve with neurological symptoms, such as dysautonomia, dyskinesia (face, mouth, tongue and limbs)[6], hypoventilation, hyperthermia, ataxia, decreased responsiveness, seizures and coma[1,2,4,6,7].
The pattern of young female patient with neuropsychiatric symptoms and no improvement with antipsychotic drugs must lead doctors to investigate underlying causes, including tumors. Investigation must include: (1) medical history: these patients frequently have no neuropsychiatric history, but a prodrome of flu-like symptoms two weeks earlier, with headache, nausea and fatigue, is present in 70% of the cases[4,7]; (2) CSF and serum search for antibodies[8] and (3) image exams, such as ultrasound, CT or MRI[9].
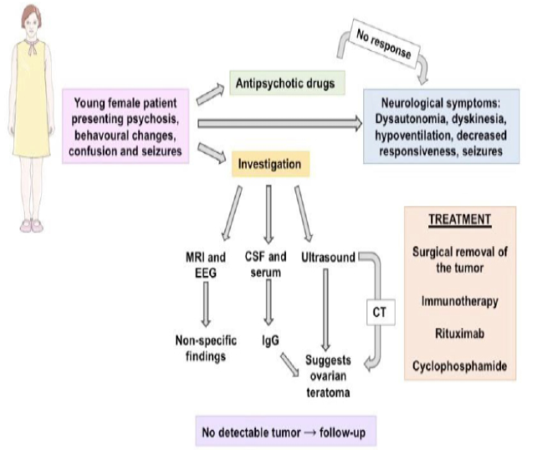
Figure 1: Typical epidemiology (young female patient), presentation and progression of the case.
Woman’s image obtained from Smart Servier
Physiopathology
NMDA receptors are ligand-gated ion channels involved in cognitive processes such as behavior, memory, learning and plasticity[5,10]; and are mostly found in frontotemporal region and hippocampus[1,11]. The pathophysiology of anti-NMDAR encephalitis is strongly associated with its symptoms[12], and most of these symptoms can be mimicked by using NMDA antagonists like ketamine[11,12], suggesting that anti-NMDAR encephalitis is a reversible disorder caused by hypofunction of NMDA receptors[5].
Antibodies attachment to GluN1 subunit (glycine-binding subunit) of the NMDAr causes internalization of the receptor in synaptic sites, resulting in loss of the connection between NMDAr and ephrin B2 receptor (EphB2Rs)[12-14]. EphB2Rs plays a role stabilizing NMDAr in the postsynaptic membrane and providing plasticity[10]. Internalization occur both in excitatory and inhibitory pathways and reduce NMDA dependent synapses[10].
Besides this mechanism, Lynch et al. suggests that IgG also acts primarily as NMDA agonist before inducing its hypofunction and potentially causes a transition from synaptic to extra synaptic receptors. NMDA receptors over activity is a possible explanation to the connectivity disruption and symptoms like seizures, a non-logical feature of pure hypofunction disorder of NMDA[5,11].
Connectivity disruptions provide a solid explanation for psychotic symptoms and memory loss. Similar evidences of connectivity imbalance are demonstrated in disturbances like schizophrenia and psychosis[15].
In a recent study, Chefdeville et al. demonstrated the prevalence of 19.9% (57/286) of ovarian teratoma in 286 cases of anti-NMDA receptor encephalitis. 27/57 of these teratoma cases were analyzed and 96% (26/27) had neural tissue, compared to 38% from control group[16]. All these 26 teratomas had immune infiltrated close to the neural-glial tissue, supporting the idea that germinal-center-like structures inside the teratomas could be associated with antibodies production, at least in the beginning of the disease. This hypothesis is corroborated by the fact that IgM antibodies were found in patients’ serum even 6 months after the onset[17]. The progression to an intrathecal production of antibodies is probably caused by a break in the blood brain barrier, that could be explained clinically by the viral prodrome which occur in most cases[4].
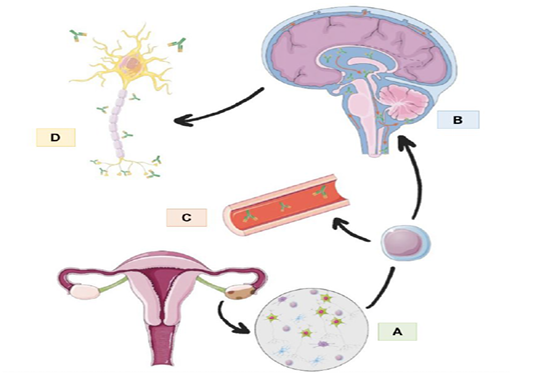
Figure 2: The image summarized the pathophysiology of anti-NMDA encephalitis associated with ovarian teratoma. A: teratoma tissue expression NMDA antigens, with lymphocyte infiltration and initial production of antibodies. B: the migration of activated B cells to the Central nervous system where they produce antibodies against NMDA intrathecally. C: the antibodies against NMDA receptor, produced in the germ cell like tissue on the teratoma, found in serum. D: Antibodies acting all along the neuron in the NMDA receptors, with a specially important action in synaptic receptors. Uterus, B cell, SNC and neuron images obtained from Smart Servier
Ovarian Teratoma
Dalmau et al reported the prevalence of identified tumor of 59% in patients with anti-NMDA receptor encephalitis. 97% of the patients with tumor were female; and, in 95% of these female patients, the tumor was ovarian teratoma[4,5]. Therefore, ovarian teratoma may be defined as the major cause of anti-NMDA receptor encephalitis.
Mature cystic teratoma, also known as dermoid cyst, is a benign germ cell neoplasm that arises from totipotent cells of the ovary[18]. Although malignant ovarian teratoma may happen in 5% of the cases, dermoid cyst is the most prevalent form found in anti-NMDAR encephalitis. Neurological presentation is the same for both malignant or benign forms, but mortality is higher in the malignant tumors[19].
Since these tumors arise from totipotent cells, tissue from the three layers (ectoderm, mesoderm and endoderm) may develop, including nerve tissue[18]. Ovarian teratomas containing nervous tissues may lead to anti-NMDAR encephalitis, even the small ones[1]. Iemura et. al demonstrated ovarian teratomas with median size of 5 cm, varying from 2.5 cm to 15 cm, in patients with encephalitis[20]. In 10% of the cases, dermoid cysts are bilateral[18], synchronous or not[5].
In patients with encephalitis, histopathological study of the nervous tissue demonstrates neurons densely aggregated occasional mitosis and positive immunohistochemistry reaction for NMDA receptor[5,20]. In ultrasound images, typical appearance of mature teratoma is cystic adnexal unilocular mass[18]. Diagnosis of ovarian teratoma may happen after the encephalitis recovery[5,21], and therefore these patients must me followed up.
Diagnosis
Antibodies (IgG) against NMDA receptors may be found in serum, CSF or both[22]. Brain MRI and EEG may be either normal or show non-specific findings of increased signal (cerebral cortex, cerebellum, hippocampi, medial temporal lobe) and disorganized activity (slow delta waves), respectively[4,5,22].
When limbic system is involved, Gultekin’s et al criteria for paraneoplastic limbic encephalitis diagnosis may be useful[23].
Table 1: Diagnosis of paraneoplastic limbic encephalitis by Gultekin
|
I. A compatible clinical picture |
III. Exclusion of other neuro-oncological complications |
|
II. An interval of < 4 years between the development of neurological symptoms and tumour diagnosis |
IV. At least one of the following: CSF with inflammatory changes but negative cytology, MRI demonstrating temporal lobe abnormalities or EEG showing epileptic activity in the temporal lobes |
Ultrasound is an easy and appropriate exam to investigate ovarian teratomas. It can be done both transvaginal and transrectal and reveal high echogenic images[22]. CT may be useful in inconclusive cases[9]. Treatment may be instituted before the final diagnosis, according to the severity and progression of the case[24].
Despite the search, 41% of the patients with anti-NMDA receptor encephalitis do not have detectable tumors[5]. Ovarian teratomas may be too small and difficult to detect in image exams[9] or may develop after the paraneoplastic syndrome. Therefore, it is recommended to repeat exams in young women with no detectable tumors, every year, for two or three years[10].
Pregnant women with history of anti-NMDA receptor encephalitis may be monitored. Maternal-fetal transfer of anti-NMDAR antibodies may occur in both symptomatic and asymptomatic women, and the consequences for the baby are not established[25].
Differential diagnosis includes infectious encephalitis (especially herpes simplex encephalitis), psychiatric disorders, psychotropic drugs use, meningitis, systemic autoimmune diseases, MELAS syndrome, prion diseases and Hashimoto’s encephalopathy[4,10,26,27].
Methods and Results
We have made a non-systematic review on PubMed with the descriptors “ovarian teratoma and encephalitis” and “anti- NMDA encephalitis”. No year restriction was applied. The articles were selected in consensus between the authors.
Treatment
Best therapeutic results are obtained with the combination of surgical removal of the tumor and immunotherapy, such as IVIG, corticosteroids (methylprednisolone) and plasmapheresis[1,7,9,11]. In some cases, rituximab and cyclophosphamide may be required[11]. 75% of the patients have full recovery or mild neurological deficits, while 25% may have severe residual deficits or die[1,7]. Intensive care is often required for ventilation support and autonomic instability management, even before the diagnosis[1,6].
Among other paraneoplastic encephalitis, anti-NMDA receptor encephalitis carries a good prognosis[23], and the reversibility of neurological symptoms is possible due to the immune mediated neuronal dysfunction[4]. Recovery is usually slow[5]. Clinical improvement happens in parallel to the decrease of antibodies levels[5]; although this information is not enough to guide clinical decisions and antibodies may be present for months in serum and CSF after recovery[10]. Because of the important role of NMDA receptors in learning and memory abilities, persistent amnesia of the whole process may occur after recovery[5].
In patients with no detectable tumors, recovery may be slower, and relapses may be more frequent[27]. These patients must be followed up after recovery because tumors may be detected after the paraneoplastic presentation[5,21].
Conclusion
In conclusion, anti-NMDAR encephalitis associated with ovarian teratoma is a paraneoplastic condition affecting mostly young women in their most productive phase of life. Once early resection of the tumor and early institution of immunotherapy are associated with better outcomes, neurologist and clinicians should be aware of young women presenting with new onset psychiatric or neurological symptoms even when tumor is not found in image studies.
Sources of Support: No funding supports
Conflict of interest: All authors declare that there are no conflicts of interest
Authors contributions: All authors contributed equally for the article
References
- 1. Abdul-Rahman, Z.M., Panegyres, P.K., Roeck, M., et al. Anti-N-methyl-D-aspartate receptor encephalitis with an imaging-invisible ovarian teratoma: a case report. (2016) J Med Case Rep 10(1): 296.
- 2. Barth, A., Nassenstein, I., Tröbs, R.B., et al. N-Methyl-D-Aspartate Receptor Encephalitis with Psychiatric Symptoms and an Ovarian Teratoma Detected by MRI in a 17-Year-Old Girl. (2019) Neuropediatrics 50(4): 253–256.
- 3. Hegen, H., Uprimny, C., Grams, A., et al. Bi-insular cortical involvement in anti-NMDA-receptor encephalitis - a case report. (2016) BMC Neurol 16: 130.
- 4. Jandu, A.S., Odor, P.M., Vidgeon, S.D. Status epilepticus and anti-NMDA receptor encephalitis after resection of an ovarian teratoma. (2016) J Intensive Care Soc 17(4): 346–352.
- 5. Dalmau, J., Gleichman, A.J., Hughes, E.G., et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. (2008) The Lancet Neurol 7(12): 1091–1098.
- 6. Acién, P., Acién, M., Ruiz-Maciá, E., et al. Ovarian teratoma-associated anti-NMDAR encephalitis: a systematic review of reported cases. (2014) Orphanet J Rare Dis 9: 157.
- 7. Ahmad, J., Sohail, M. S., Khan, A., et al. Anti-n-Methyl-d-Aspartate-Receptor (NMDAR) Encephalitis in Association with Ovarian Teratoma. (2017) Cureus 9(7): e1425.
- 8. Herman, L., Zsigmond, I. R., Peter, L., et al. Anti-NMDA (N-metil-D-aszparaginsav) receptor enkefalitisz: irodalmi összefoglaló, esetismertetés és kutatási terv [Review of the psychiatric aspects of anti-NMDA (N-methyl-D-aspartic acid) receptor encephalitis, case report, and our plans for a future study]. (2016) Neuropsychopharmacol Hung 18(4): 199–208.
PubMed│CrossRef│Others
- 9. Chiu, H.C., Su, Y.C., Huang, S.C., et al. Anti-NMDAR encephalitis with ovarian teratomas: Review of the literature and two case reports. (2019) Taiwanese J Obstet Gynecol 58(3): 313–317.
- 10. Guasp, M., Dalmau, J. Encephalitis associated with antibodies against the NMDA receptor. (2018) Med Clín (Barc) 151(2): 71–79.
- 11. Lynch, D.R., Rattelle, A., Dong, Y.N., et al. Anti-NMDA Receptor Encephalitis: Clinical Features and Basic Mechanisms. (2018) Adv Pharmacol 82: 235–260.
- 12. Dalmau, J. NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture. (2016) Neurology 87(23): 2471–2482.
- 13. Moscato, E.H., Peng, X., Jain, A., et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. (2014) Ann Neurol 76(1): 108–119.
- 14. Venkatesan, A., Adatia, K. Anti-NMDA-Receptor Encephalitis: From Bench to Clinic. (2017) ACS Chem Neurosci 8(12): 2586–2595
- 15. Peer, M., Prüss, H., Ben-Dayan, I., et al. Functional connectivity of large-scale brain networks in patients with anti-NMDA receptor encephalitis: an observational study. (2017) The Lancet Psychiatry 4(10): 768–774.
- 16. Chefdeville, A., Treilleux, I., Mayeur, M.E., et al. Immunopathological characterization of ovarian teratomas associated with anti-N-methyl-D-aspartate receptor encephalitis. (2019) Acta Neuropathol Commun 7(1): 38.
PubMed│CrossRef│Others
- 17. Makuch, M., Wilson, R., Al-Diwani, A., et al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: Therapeutic implications. (2018) Ann Neurol 83(3): 553–561.
- 18. Multani, J., & Kives, S. Dermoid cysts in adolescents. (2015) Curr Opinion Obstet Gynecol 27(5): 315–319.
- 19. Bost, C., Chanson, E., Picard, G., et al. Malignant tumors in autoimmune encephalitis with anti-NMDA receptor antibodies. (2018) J Neurol 265(10): 2190–2200.
- 20. Iemua, Y., Yamada, Y., Hirata, M., et al. Histopathological characterization of the neuroglial tissue in ovarian teratoma associated with anti-N-methyl-D-aspartate (NMDA) receptor encephalitis. (2018) Pathol Int 68(12): 677–684.
- 21. Omata, T., Kodama, K., Watanabe, Y., et al. Ovarian teratoma development after anti-NMDA receptor encephalitis treatment. (2017) Brain Dev 39(5): 448–451.
- 22. Gultekin, S.H., Rosenfeld, M.R., Voltz, R., et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. (2000) Brain 123(Pt 7), 1481–1494.
- 23. Yan, B., Wang, Y., Zhang, Y., et al. Teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis: A case report and literature review. (2019) Medicine 98(21): e15765.
- 24. Braverman, J.A., Marcus, C., Garg, R. Anti-NMDA-receptor encephalitis: A neuropsychiatric syndrome associated with ovarian teratoma. (2015) Gynecol Oncol Rep 14: 1–3.
- 25. Chourasia, N., Watkins, M.W., Lankford, J.E., et al. An Infant Born to a Mother With Anti-N-Methyl-d-Aspartate Receptor Encephalitis. (2018) Pediatr Neurol 79: 65–68.
- 26. Kattepur, A.K., Patil, D., Shankarappa, A., et al. Anti-NMDAR limbic encephalitis--a clinical curiosity. (2014) World J Surg Oncol 12: 256.
- 27. Zhang, W., Yan, L., Jiao, J. Repeated misdiagnosis of a relapsed atypical anti-NMDA receptor encephalitis without an associated ovarian teratoma. (2017) Neurosci Lett 638: 135–138.












