Antibacterial and Antifungal Activities of Zinc-Silicon Oxides Nanocomposite
Muhammad Arshad, Sadia Haneef, Nabeela Aslam, Amna Afzaal
Affiliation
Department of Chemistry, Nano-ChemistryLab, GC University Lahore, Pakistan
Corresponding Author
Farrukh, M.A. Department of Chemistry, Nano-ChemistryLab, GC University Lahore, 54000, Pakistan; E-mail: akhyar100@gmail.com
Citation
Farrukh, M.A. et.al. Antibacterial and Antifungal Activities of Zinc-Silicon Oxides Nanocomposite. (2016) Lett Health Biol Sci 1(1): 5- 9.
Copy rights
© 2016 Farrukh, M.A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Nanocomposite; Deposition-precipitation; Antibacterial activity; Antifungal activity
Abstract
Synthesis of ZnO-SiO2 nanocomposite was carried out via deposition-precipitation method by using acetonitrile as solvent. Synthesized nanocomposite was characterized by using different analytical techniques like Fournier Transformation Infrared Spectroscopy (FT-IR), Thermo-Gravimetric Analysis (TGA), Powder X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Dynamic Light Scattering (DLS). Biological potential such as antibacterial and antifungal activities were also studied by using Disc Diffusion method and Agar Well Diffusion Assay respectively. The particle size of ZnO-SiO2 nanocomposite calculated by TEM was found to be 6.2 nm. The nanocomposite showed better antibacterial activity than antifungal activity.
Introduction
Several studies have suggested that nanoparticles can be used as efficient antimicrobial agents. Zinc oxide powder has been used as an active constituent in ointment, creams and lotions for skin treatments owing to its antibacterial properties. However, zinc oxide nanoparticles are much more effective in suppression of microorganism growth. Furthermore, at nanoscale the properties of particles are widely changed, enhancing their semiconducting abilities as well as the efficacy in inhibiting the growth of bacteria[1]. Microorganisms are the major constituents in the biosphere and their growth may be implement beneficial or harmful effect on the environment regarding to the human being. They are naturally symmetrical with environment and human body. So it is necessary to control their harmful effects by inhibiting their growth. Antimicrobial agents are chemical compounds which have potential to inactivate or inhibit the growth of microorganism[2]. These antimicrobial agents have vast applications in various fields including food preservation and packaging, medicine, water disinfection, textile fabrics and hospital implants[3].
Nanotechnology is also getting interest due to their attractive material properties and applications in various fields such as photo-catalysis, organic pollutants, optical devices, antibacterial coatings and sensors[4]. Nanotechnology is getting advancement particularly the potential to prepare metal oxide nanomaterials of specific shape and size which leads it in the development of new antibacterial agents[5].
Various nanoparticles of metal oxides have been synthesized and found good inhibitor of different bacterial strains. Activity of nanoparticles is directly dependent on the bacterial strains[6]. Metal oxides have wide range applications in sensors, microelectronic circuits, fuel cells and catalysis. The unique properties of metal oxides are due to their limited size and high density of edge surface sites[7]. The metal oxide nanoparticles such as magnesium oxide (MgO), gold (Au), titanium dioxide (TiO2), calcium oxide (CaO), silicondioxide (SiO2), copper oxide (CuO) and silver oxide (Ag2O), are published as antimicrobial potential[8]. The antimicrobial potential of metal oxides nanoparticles has been recognized to their smaller size and higher surface to volume ratio, which enables them to closely bind with microbial strain but it does not due to the discharge of metal ions in solution[9].
Different methods have been used to synthesize nanoparticles like sol-gel method, surfactant mediated method[10], deposition-precipitation method, anodization method, wet-oxidation method[1], microwave-assisted combustion, thermal evaporation template methods[11], electrodeposition and sonication[12]. Metal oxide nanoparticles synthesized by any of these mentioned methods are used against bacterial strains by adopting various protocols[6]. The catalytic activity and antimicrobial properties of nanoparticles can be enhanced by doping the nanoparticles with various methods[1].
For a long time, scientist used antimicrobial drugs to inhibit and kill bacteria or other microbes but however they have been developed a specific microbial resistance over time. So one of the most promising strategy to get through this microbial resistance was to use nanoparticles[13]. Interestingly, ZnO-NPs are reported by several studies as non-toxic to human cells. This aspect necessitated their usage as antibacterial agents, noxious to microorganisms, and hold good biocompatibility to human cells. The various antibacterial mechanisms of nanomaterials are mostly attributed to their high specific surface area-to-volume ratios[14].
In this work we have synthesized ZnO-SiO2/ nanocomposites via deposition-precipitation method by using acetonitrile solvent and tartaric acid as a stabilizing agent. Their antibacterial activity against Bacillus subtilis (gram positive) and E. Coli (gram negative) strains and antifungal activity against Candida parapsilosis and Aspergilus niger was determined. The major purpose of this study was to investigate the anti-bacterial and anti-fungal effects of nanocomposite against various strains. SiO2 was used to increase the photo-catalytic activity while ZnO was used to eliminate harmful bacteria[15].
Experimental
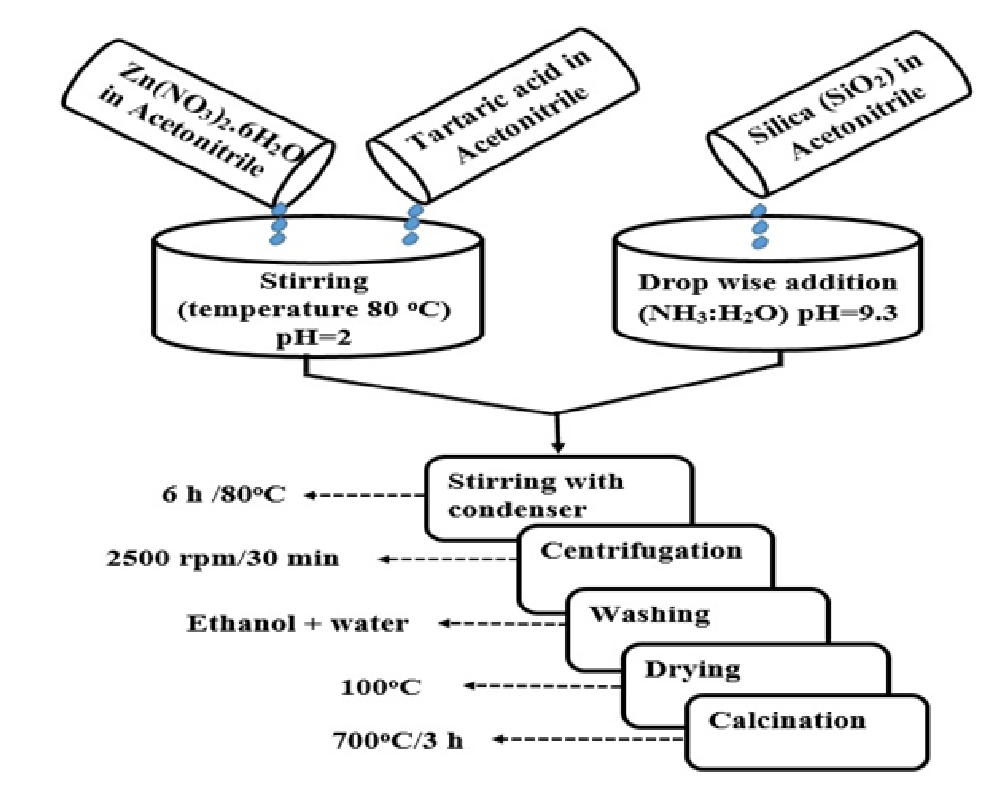
Synthesis of ZnO-SiO2 nanocomposite was carried out via deposition-precipitation method by using acetonitrile solvent. The chemicals used for experimental work include Zinc Nitrate Zn(NO3)2.6H2O (Merck), Tartaric acid (Panreac), liquid Ammonia (Biom), Ethanol (Biom), Silicon dioxide (DAEJUNG), Acetonitrile (PANREAC). The analytical instruments used for experimental work were Analytical Balance (Shimadzu), , FTIR (Shimadzu), XRD (Philips X’ Pert), FESEM (JEOL 7600), TEM (JEOL JEM 1010), TGA/SDT (G600 V8.3 Build 101), Furnace (VULCAN D-550), Oven (EV 108 Ac–Kapa), pH meter (Chemcadet 5986-62), Centrifuge machine (Sigma 1-14), DLS.
Synthesis of ZnO-SiO2 nanocomposite
ZnO-SiO2 nanocomposite was synthesized by using equimolar concentration of zinc nitrate Zn(NO3)2.6H2O (0.2M) and tartaric acid (0.2M), each of them was dissolved separately in 10 mL of acetonitrile. After mixing both solutions, stirred the solution for 20 min (Ph = 2) at an elevated temperature 80°C. Now add 0.5g of SiO2 in the 20 mL of acetonitrile in a separate beaker and stirred for 20 min (pH = 7.2). Adjust the pH by dropwise addition of NH3 : H2O in the prepared solution of SiO2 (pH = 9.3). Now mix the both solution. The suspension was refluxed for 6 h at 80°C and then centrifuged at 2500 rpm for 30 min. The precipitates were separated and washed several times with water and ethanol and dried at 100°C in the oven. The dried precipitate was annealed at 700°C for 3 h.
Scheme 1: Schematic diagram of ZnO-SiO2 nanocomposite
Antibacterial activity
Antibacterial activity of ZnO-SiO2 nanocomposite was studied against two bacterial strains Bacillus subtilus and Escherichia coli.
Preparation of fresh bacterial culture
Nutrient broth (Oxoid, UK) 100 mL was prepared in Erlenmeyer flask contained glass beads. pH was adjusted to 7.4 by addition of buffer solution and autoclaved at 121°C for 15 min. Mixture was allowed to cool in laminar air flow. Broth was inoculated with 100 μL bacteria form stored bacterial culture. Growth medium was incubated in shaker at 37°C for 24 h to get 5 × 109 cells per mL.
Disc diffusion method
Nutrient agar was prepared by mixing agar medium in distilled water. Agar medium and 10 mm discs of wicks paper were sterilized in auto- clave and allowed to cool in laminar air flow. The agar medium was inoculated with 50 μL fresh bacterial culture and sterilized discs were poured with synthesized nanocomposite and spread in petri plates with positive control in the center. Incubate the petri dishes at 37°C for 24 h. Zones were measured with zone reader. Experiment was performed with both bacterial strains.
Antifungal activity
The antifungal activity was performed according to Devi, et al., 2014[16] with slight modification. Antifungal activity of the synthesized ZnO-SiO2 nanocomposite in acetonitrile solvent was determined using the agar well diffusion assay. Stock cultures of Candida parapsilosis and Aspergilus niger were prepared and maintained in Sabouraud Dextrose Agar (SDA) slants at 4ºC. A positive control drug (Nystatin) was also done parallel. The plates were examined for evidence of zone of inhibition, which appear as area around the walls. The diameter of such zones of inhibition was measured using a meter ruler. Mean value was calculated by performing the experiments in triplicates.
Results and Discussion
Fourier Transform Infrared Spectroscopy (FT-IR)
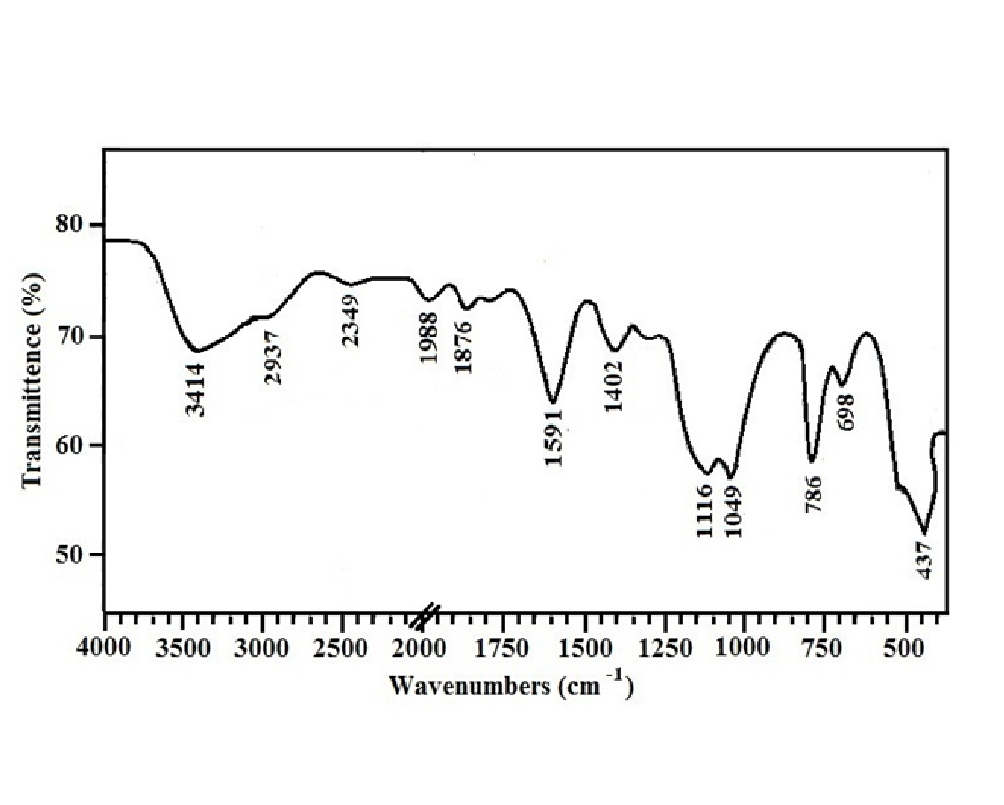
Figure 1 shows the FT-IR spectrum of ZnO-SiO2 nanocomposite. The broad absorption band at 3000-3600 cm-1 and the peak at 1591 cm-1 can be assigned to the stretching and bending vibrations of OH group of H2O molecule respectively. The presence of these peaks in the spectrum was attributed the adsorption of atmospheric water on surface of nanocomposite during FTIR measurements. The small absorption peak at 2937 cm-1 is due to the symmetric vibrations of CH group of tartaric acid which was used as a stabilizing agent[17]. The absorption peak at 2349 cm-1 is the C=O stretching mode vibrations which is indicating the adsorption of atmospheric CO2 on nanocomposite’s surface[18]. The peak at 1988 cm-1 is due to stretching vibrations of C-N bond[19]. The absorption peaks at 1402 and 1876 cm-1 are the stretching vibrations of C-O and C=O bonds of carboxyl group respectively. The absorptions at 1049 cm-1 and 1116 cm-1 are due to dissymmetric vibrations of Si-O-Si[20] and the absorption peak at 788 cm-1 is assigned to Si-OH. The absorption peak at 698 cm-1 shows the stretching vibrations of Si-O bond[17]. The absorption peak at 437 cm-1 is attributed to stretching vibrations of Zn-O[18].
Figure 1: FT-IR spectra of 0.1M ZnO-SiO2 nanocomposite
Powder X-Ray Diffraction (XRD)
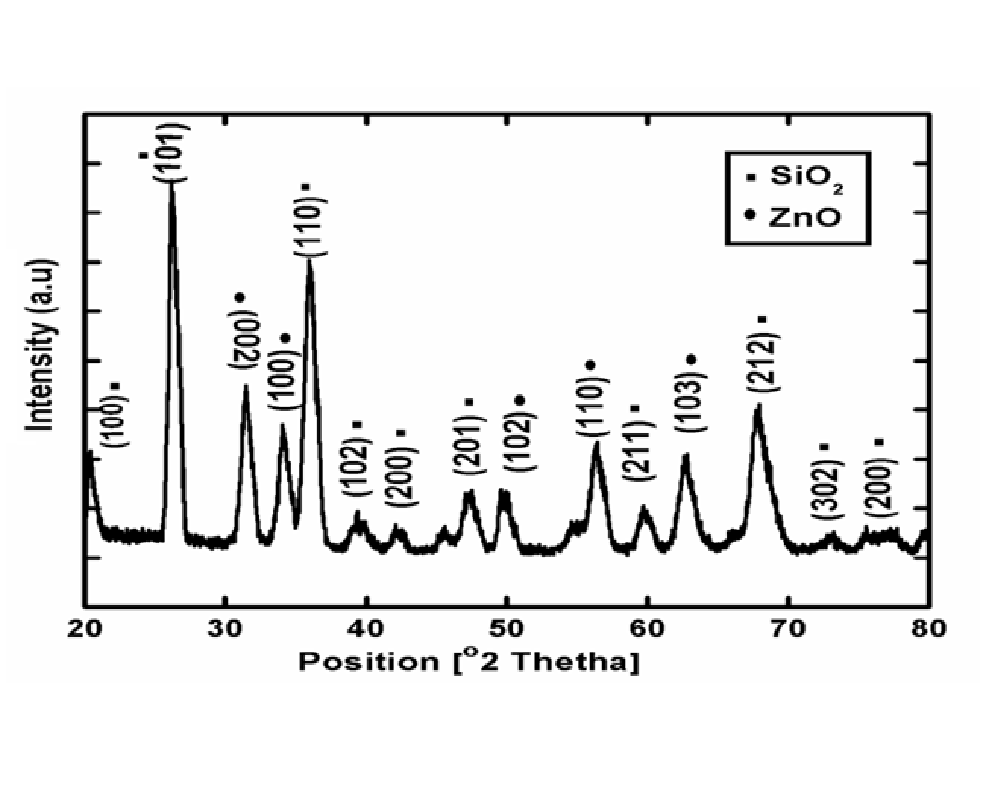
Figure 2 shows the XRD pattern of 0.1M ZnO-SiO2 nanocomposite synthesized by deposition-precipitation method in Acetonitrile. It can be seen from the figure that all the proposed samples are well indexed to the hexagonal phase of SiO2 with reference code (01-089-8939). ZnO-SiO2 nanocomposite synthesized in Acetonitrile shows the characteristics peaks of SiO2 at 2θ = 20.934°, 26.789°, 36.191°, 39.489°, 42.30°, 45.59°, 54.77°, 60.057°, 67.882°, 73.90°, 75.36°, with diffraction planes (100), (101),(110), (102), (200), (201), , (211), (212), (302), and (200) which are well indexed with [ICSD PDF00-001-0649] and [ICSD PDF database 00-001-0649] with Quartz structure.
The ZnO-SiO2 in addition to above diffraction peaks, some characteristics peaks of zinc oxide (ZnO) is also shown which are all well indexed with [ICSD PDF database 01-079-0208] with hexagonal structure at 2θ = 31.74, 34.31, 36.31, 50.42, 56.58 and 62.25 with diffraction peaks at (002), (100), ( 102), (110) and (103) respectively. XRD pattern was analyzed to determine the peak intensity, position and width.
Figure 2: XRD pattern of ZnO-SiO2 nanocomposite synthesized in Acetonitrile solvent
Scanning Electron Microscopy (SEM)
Figure 3 shows the SEM analysis of ZnO-SiO2 nanocomposite synthesized in acetonitrile. The micrograph shows coalesced flake-like mass. From the figure it can be deduced that ZnO-SiO2 nanocomposite has small dimension and the structure becomes more detained. The SEM micrograph shows that the larger particles mostly consist of silicon and oxygen and the flakes are mostly made up of zinc and oxygen. The ZnO-SiO2 nanocomposite shows the spherical morphology with highly porous structure.
Figure 3: SEM image of ZnO-SiO2 nanocomposite
Transmission Electron Microscopy (TEM)
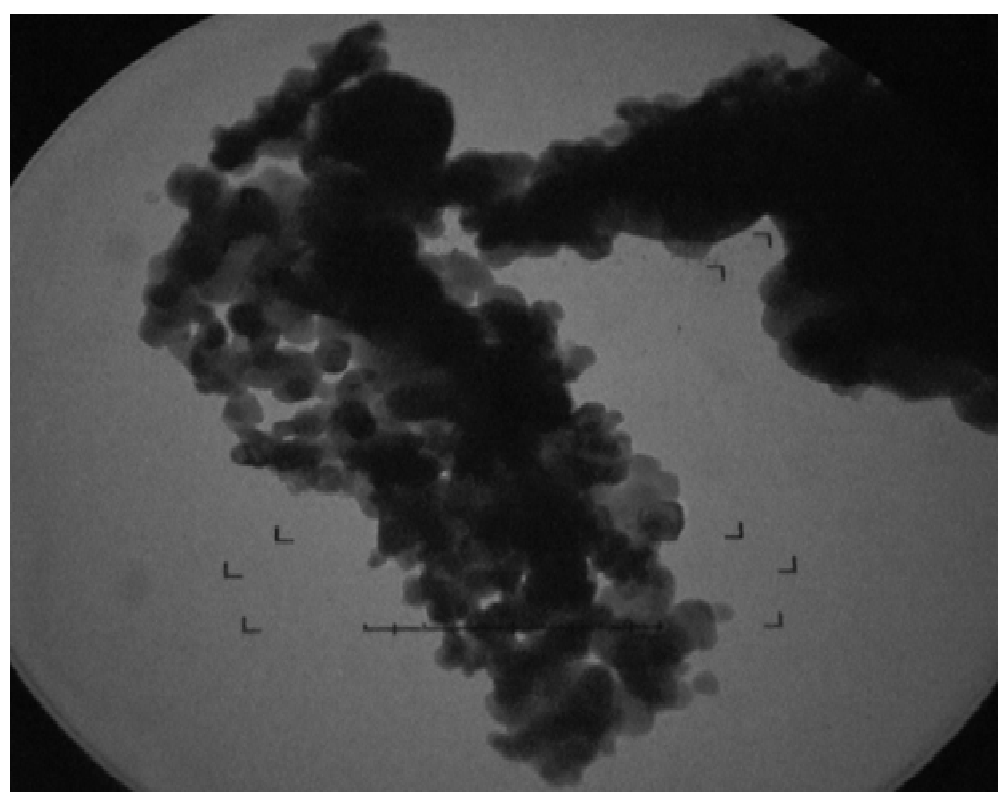
To elucidate the size of ZnO-SiO2 nanocomposite, TEM image was taken with JEOL JEM 1010. The image shows that ZnO is fused on the surface of SiO2 uniformly. Figure 4 generates some amorphicity and agglomeration. These clusters create effect on both the porosity and on surface area of SiO2, size of image was calculated by the following formula.
Size of image = Object size × Magnification power
The average particle size calculated was 6.2 nm.
Figure 4: TEM image of ZnO-SiO2 nanocomposite
Thermo Gravimetric Analysis (TGA)
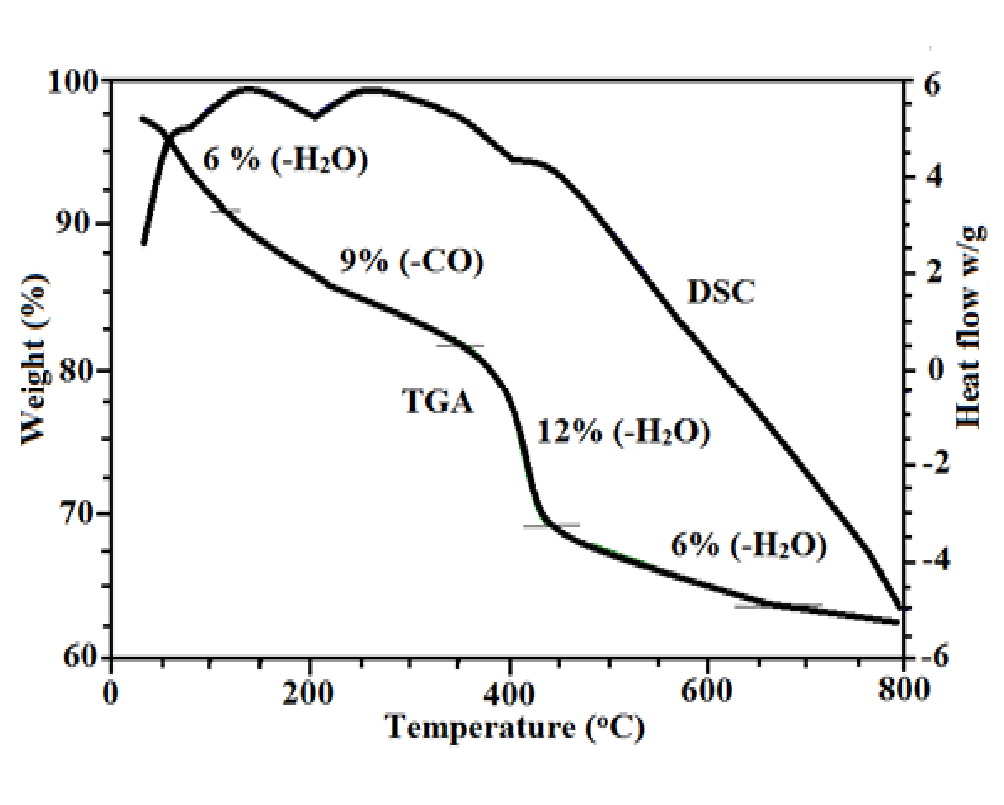
The TGA-DSC curve represents weight loss of sample as a function of temperature for ZnO-SiO2 nanocomposite shown in Figure 5. Four major weight losses were clearly evident. The first step involves the weight loss of 6% at 100°C which is due to removal of physically adsorbed water molecule. The second curve indicates 9% weight loss which is due to removal of carbon monoxide from tartaric acid at 300°C. Third major weight loss was 12% due to removal of two water molecules which elucidate the decomposition of zinc tartrate to ZnO[21]. While weight loss at 700°C elucidated the temperature above which the vicinal hydroxyl group of silica were completely condensed[22].
Figure 5: TGA/DSC curve of ZnO-SiO2 nanocomposite
Dynamic Light Scattering (DLS)
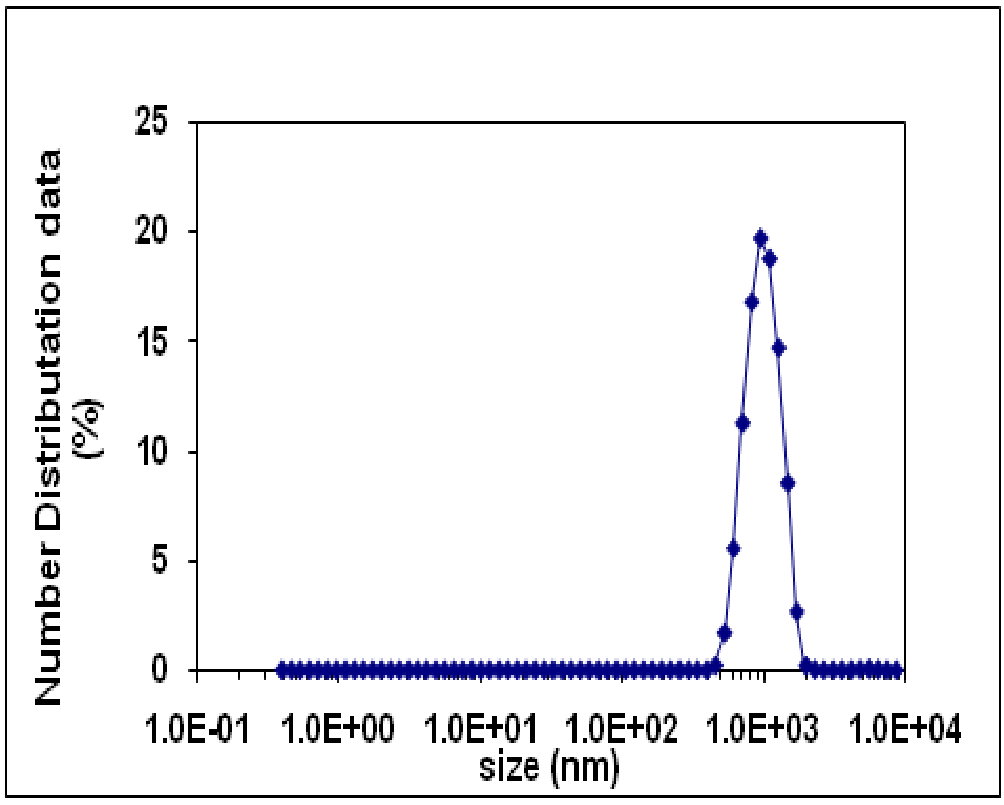
Dynamic Light Scattering (DLS) is an important instrument for characterizing the size of nanoparticles in solution. DLS deals with the light scattered from a laser that passes through a colloidal solution and by analyzing the modulation of the scattered light intensity as a function of time, the hydrodynamic size of particles and particle cumulation can be determined. DLS analysis is used to determine the hydrodynamic diameter of synthesized nanoparticles. Hydrodynamic diameter of the nanoparticles is always larger than their size due to presence of stabilizer at their surface. Figure 6 shows the number distribution of ZnO-SiO2 nanocomposite synthesized by precipitation method by using acetonitrile solvent. The result clearly shows that the Z-Average value as the mean value of the hydrodynamic diameter is 19.6 nm and Peak-Intensity is 955. A second important number is the polydispersity index which is a measure of the width of the particle size distribution. Polydispersity indices less than 0.1 are typically referred to as “monodisperse”. PDI (0.06) is calculated by using following formula[23].
Figure 6: DLS spectra of 0.1M ZnO-SiO2 nanocomposite
Biological Applications
Antibacterial activity of ZnO-SiO2 nanocomposite
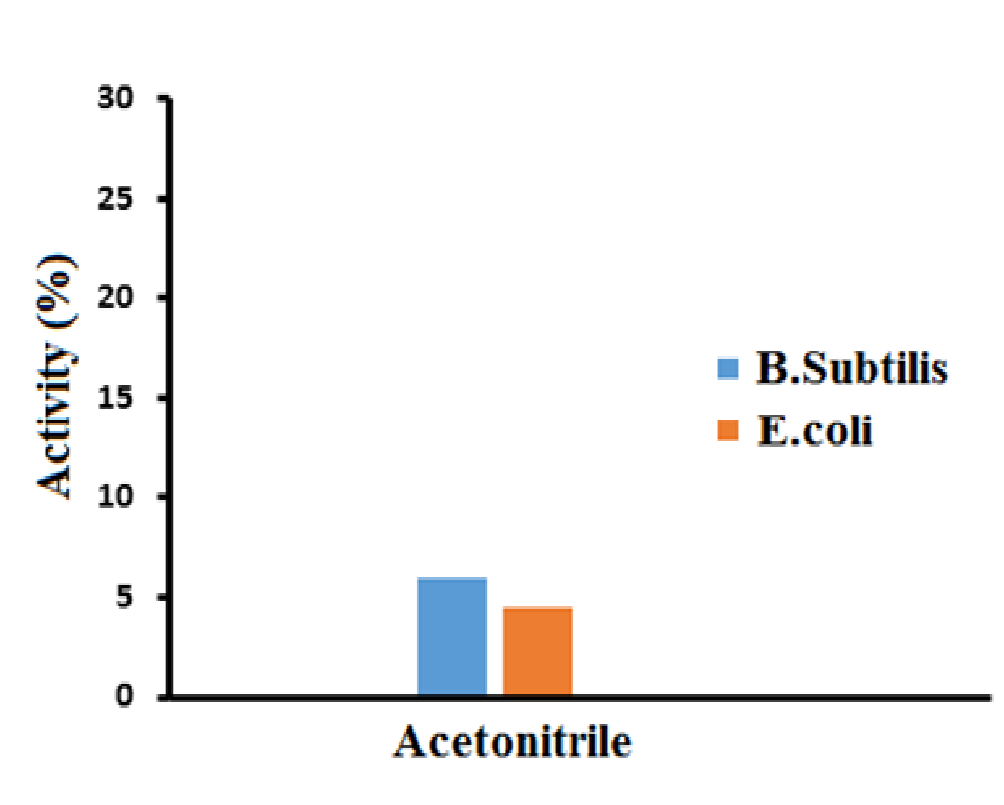
To confirm the antibacterial activity of ZnO-SiO2 nanocomposite, the growth inhibition of the two bacterial strains i.e. Bacillus subtilis and E. coli was investigated by Disc diffusion method. The prepared nanocomposite showed 6% activity against Bacillus subtilis and 4.5% activity against E. coli, as shown in the Figure 7.
Figure 7: Antibacterial activity of ZnO-SiO2 nanocomposite
Antifungal activity of ZnO-SiO2 nanocomposite
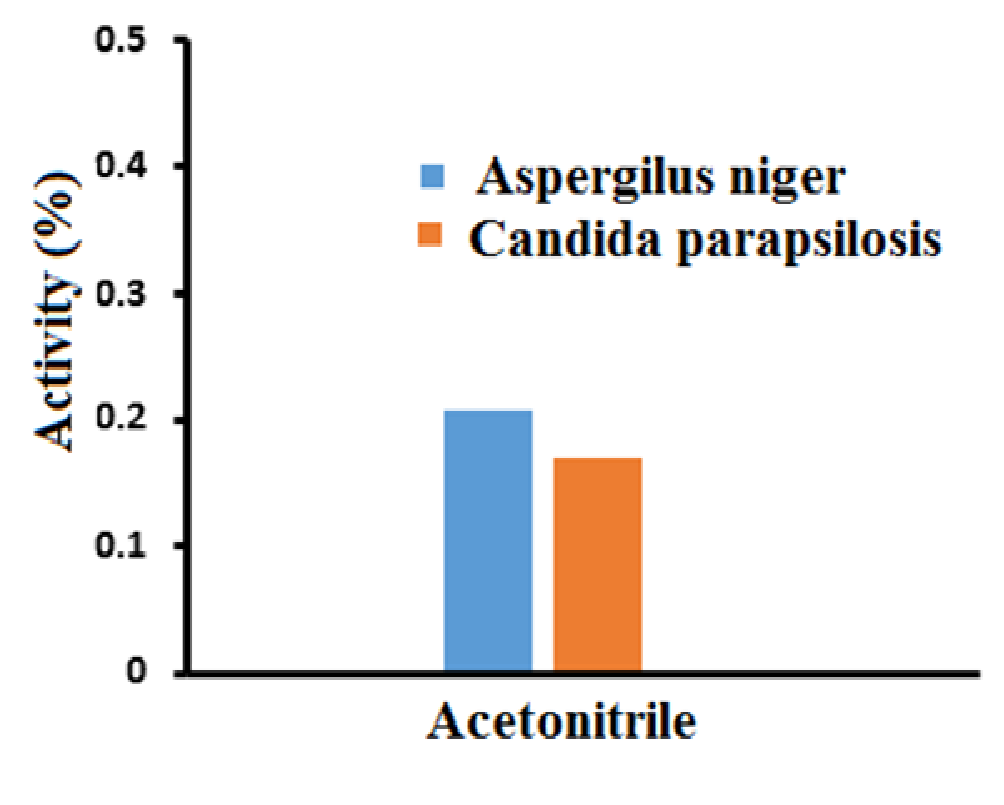
The antifungal activity of ZnO-SiO2 nanocomposite was performed with an average diameter of 25 nm against the tested yeasts diluted in Mueller Hinton Broth to the final concentrations of silicon dioxide. The obtained results showed that the ZnO-SiO2 nanocomposite inhibited some of the tested Candida parapsilosis and Aspergilus niger. At very low concentrations the inhibition was dependent on the yeast species tested. The lowest MIC of ZnO-SiO2 nanocomposite showed the antifungal activity at the concentration of 0.207 mg/L against Aspergilus niger and 0.17 mg/L against Candida parapsilosis as shown in Figure 8.
Figure 8: Antifungal activity of ZnO-SiO2 nanocomposite
Conclusion
The purpose of present research work was to synthesize the ZnO-SiO2 nanocomposite in acetonitrile solvent by deposition precipitation method. Different characterizations were carried out to confirm the size and shape of the prepared nanocomposite. ZnO-SiO2 nanocomposite showed better antibacterial activity against Bacillus subtilis than E. coli and better antifungal activity against Aspergilus niger than Candida parapsilosis. Acetonitrile solvent showed greater antibacterial activity than antifungal activity.
Acknowledgement: The authors would like to highly acknowledge to the Higher Education Commission Islamabad for the financial assistance to conduct this research work.
References
- 1. Arshad, M., Farrukh, M.A., Imtiaz, A., et al. Solvent Assisted Synthesis of Tin-Zinc Oxide nanoparticles: Structural Characterization and Antimicrobial Activity. (2015) Asian J Chem 27(1): 371-374.
- 2. Afzal, M., Shahid, M., Jamil, A., et al. Phytochemical Spectrum of Essential Oil of Paganumharmalaby GC-MS and Antimicrobial Activity using Sequential Solvents Fractions and Essential Oil. (2014) Asian J Chem 26: 574-578.
- 3. Li, Q., Mahendra, S., Lyon, D.Y., et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. (2008) Water Res 42(18): 4591-4602.
- 4. Ahmad,R., Mohsin, M., Ahmad, T., et al. Alpha amylase assisted synthesis of TiO2 nanoparticles: structural characterization and application as antibacterial agents. (2015) J Hazerd Mater 283: 171-177.
- 5. Azam, A., Ahmad, A.S., Oves, M., et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. (2012) Int J Nanomed 7: 6003-6009.
- 6. Ahmad, S., Farrukh, M.A., Khan, M., et al. Synthesis of Iron Oxide–Tin Oxide Nanoparticles and Evaluation of their Activities against Different Bacterial Strains. (2014) Can Chem Trans 2(2): 122-1343
- 7. Farrukh, M.A., Shahid, M., Muneer, I., et al. Influence of gadolinium precursor on the enhanced red shift of Gd/SnO2–TiO2 nanoparticles and catalytic activity. (2016) J Mater Sci-Mater El 27(3): 2994-3002.
- 8. Zarei, M., Jamnejad, A., Khajehali, E. Antibacterial effect of silver nanoparticles against four foodborne pathogens. (2014) Jundishapur J Microb 7(1): e8720.
- 9. Chwalibog, A., Sawosz, E., Hotowy, A., et al. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. (2010) Int J Nanomed 6(5): 1085-1094.
- 10. Muneer, I., Farrukh, M.A., Javaid, S., et al. Synthesis of Gd2O3/Sm2O3 nanocomposite via sonication and hydrothermal methods and its optical properties. (2014) Superlattice Microst 77: 256-266.
- 11. Perveen, H., Farrukh, A.M., Khaleeq-ur-Rehman, M., et al. Synthesis, Structural Properties and Catalytic Activity of MgO–SnO2 Nanocatalysts. (2015) Russ J Phys Chem 89(1): 99-107.
- 12. Naseem, T., Farrukh, A.M. Antibacterial Activity of Green Synthesis of Iron Nanoparticles Using Lawsonia inermis and Gardenia jasminoides Leaves Extract. (2015) J of Chem 2015: 1-7.
- 13. Spoiala, A., Albu, M.G., Ficai,A., et al. The SiO2/ZnO composite materials for cosmetic creams. (2014) Dig J Nanomater Bios 9(4): 1729-1737.
- 14. Sirelkhatim,A., Mahmud, S., Seeni, A., et al. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. (2015) Nano-Microlett 7(3): 219–242.
- 15. Mohseni, A., Maleknia, L., Fazaeli, R., et al. Synthesis TiO2/SiO2/Ag triple nanocomposite by sonochemical method and investigation of photo-catalyst effect in wastewater treatmant. (2013) Nanocon 10:16-18.
- 16. Devi, J.S., Bhimba, B.V. Antibacterial and Antifungal Activity of Silver nanoparticles Synthesized using Hypnea muciformis. (2014) BiosciBiotechnol Res Asia 11(1): 235-238.
- 17. Fanga, G., Li, H., Liua ,X. Preparation and properties of lauric acid/silicon dioxide composites as form-stable phase change materials for thermal energy storage. (2010) Mater Chem Phys 122(2-3): 533–536.
- 18. Gondal,M., Drmosh, Q., Yamani, Z., et al. Synthesis of ZnO2 nanoparticles by laser ablation in liquid and their annealing transformation into ZnO nanoparticles. (2009) Appl Surf Sci 256(1): 298–304.
- 19. Fawcett, W., Liu, G. A Study of Ion Pairing in Acetonitrile Solutions Containing Magnesium Perchlorate Using Attenuated Total Reflection FTIR Spectroscopy. (1992) J Phys Chem 4231-4236.
- 20. Li, H., Zhang, Z., Ma, X., et al. Synthesis and characterization of epoxy resin modified with nano-SiO2 and γ-glycidoxypropyltrimethoxy silane. (2007) Surf Coat Tech 201(9-11): 5269–5272.
- 21. Azam, A., Ahmed, F., Arshi, N., et al. Low temperature synthesis of ZnO nanoparticles using mechanochemical route: A green chemistry approach. (2009)Int J Theor Appl Sci 12-14.
- 22. Unger, K.K. Porous silica its properties and use as support in column liquid chromatography. (1979) Elsevier Scientlfic Publishing Company, New York 16: 7-8.
- 23. Guidelines for Dynamic Light Scattering Measurement and Analysis. (2012) NANOCOMPOSIX.COM 1(3).