Association and Common Etiology of Heart Failure and Cancer: A Systematic Review
Noman Lateef1*, Vikas Kapoor1, Junaid Ahsan1, Azka Latif1, Muhammad Adil Mumtaz1, Muhammad Zain Farooq2, Naser Yamani2, Mohsin Mirz1, Faiz Anwer3, Muhammad Hassan Dogar4
Affiliation
1Department of Internal Medicine, Creighton University Medical Center, Omaha, NE, USA
2Department of Internal Medicine, Rush University Medical Center, Chicago, IL, USA
3Department of Hematology and Medical Oncology, Cleveland Clinic, Cleveland, OH, USA
4Department of Cardiovascular Medicine, SUNY Downstate Medical Center, Brooklyn, NY, USA
Corresponding Author
Noman Lateef, Department of Internal Medicine, Resident Physician, Creighton University Medical Center, Omaha, NE, Postal address: 7500 Mercy Rd, Omaha, NE, USA, 68124, Tel: # 402-651-4961; Email: noman.mlateef@gmail.com
Citation
Lateef, N., et al. Association and Common Etiology of Heart Failure and Cancer: A Systematic Review (2019) J Heart Cardiol 4(1): 23-27.
Copy rights
© 2019 Lateef, N. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Heart failure; Cancer; Malignancy (ies); Association; Prevalence
Abstract
Background: In HF, cancer has a prevalence of 16 %, and it is identified as an independent predictor of mortality in these patients.
Objective: Evaluation of the relationship between HF and cancer.
Method: We performed an extensive database search on PubMed, Google Scholar and ScienceDirect from their inception till June, 2019 for observational studies describing the association between HF and cancer.
Results: A total of 3 cohort studies with 10,131 patients in HF-group and 49.47 million patients in non-HF group were included. The cohort studies included in our review indicated that HF is associated with increased incidence of subsequent cancer diagnosis.
Conclusion: Further clinical trials are needed to study the common etiology, association of common mechanisms behind their etiology and possible interventions for treatment to reduce poor prognosis.
Introduction
Heart failure (HF) is a significantcause of morbidity and mortality in the United States[1]. Due to ageing of the general population, increased incidence of HF and improved treatment of leftventricular (LV) systolic dysfunction and chronic HF over the last decades, the prevalence of HF has increased. A broad spectrum of concomitant disorders may complicate HF and further decrease both quality of life and clinical outcome[2,3]. Among these disorders, the prevalence of non-cardiovascular co-morbidity is very high and exceeds the prevalence of cardiovascular conditions, particularly in elderly patients[4].
Cancer is a leading cause of morbidity and mortality worldwide[5]. In HF, it has a prevalence of 16%, and it isidentified as an independent predictor of mortality in these patients[4,6]. Despite this, the available literature generally reflects a relationship between HF and associated comorbid conditions as a whole, such that the individual effects of a comorbidity on HF and its impact on HF-related outcomes remain unknown[7]. Although, a few recent studies have suggested increased risk of cancer in patients with chronic HF[8-10], the individual data is limited. Therefore, in order to further evaluate the relationship between HF and cancer, we performed an extensive database search.
Materials and Methods
Search Strategy and Data Sources
We conducted an extensive database search on PubMed, Google Scholar and ScienceDirect from their inception till June, 2019 for observational studies describing the association between HF and cancer. To identify any additional studies, the bibliographies of relevant articles were also searched. The search used the following keywords: heart failure, chronic heart failure, heart failure with reduced/preserved ejection fraction, HFrEF, HFpEF, left ventricular systolic dysfunction, and cancer(s), neoplasm(s), ‘malignancy (ies).
Study Selection
Studies were considered eligible if they met all of the following criteria: an original study comparing HF patients with an inactive control (general population or age-matched controls), reported incidence of cancers of any type, had follow-up of at least 1 year, provided hazard ratios or relative risks and corresponding 95% CIs or data to calculate them. Extra studies were identified by a hand search of all the references of the retrieved articles.
Data Extraction and Quality Assessment
Two authors (NL, VK) independently computed all the retrieved studies based on the aforementioned selection criteria. We also performed a cross-reference search of eligible articles to identify studies which we did not find in the computerized search. The following information were extracted from the studies: study design, the name of the first author; publication year; regions of study; study period; sample size; cases size; duration of follow up; gender; mean age or age range; co-morbidities and pharmacological treatment. Any disagreement was resolved through discussion and consensus.
Results
Studies and Patient Population
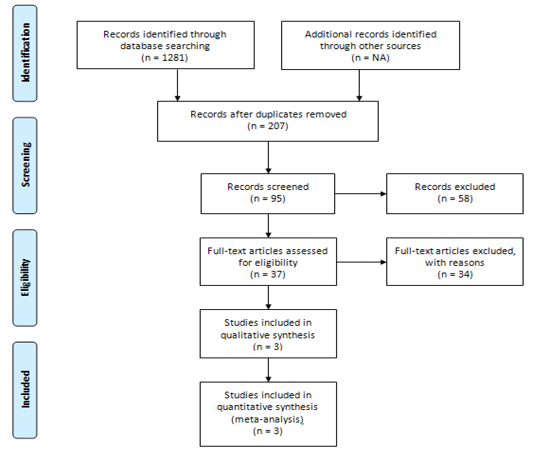
We included 3 cohort studies with 10,131 patients in HF-group and 49.47 Million patients in non-HF group[8-10]. PRISMA flow chart contains summary of this selection process. The outcome of interest was incidence of any type of cancer in each study.
In 2013 Hasin et al., conducted a prospective trial in Minnesota USA from 1979 through 2002 to assess risk of cancer among HF patients and determine its impact on survival in the community. They initially performed a case-control study with newly diagnosed HF patients (HFpEF ≥ 50%, HFrEF with EF < 50%) serving as cases (N = 961) and controls (N = 961) selected randomly from general population. Later on, the HF patients and matched controls were followed to compare long-term risk of incident cancer in both groups. They excluded non-melanoma skin cancers from study. They identified 244 new cancer cases during 9,203 person-years of follow up (mean [SD] follow-up: 7.7 [6.4] years). Cancers of Digestive system (19%, 48 out of 244) and reproductive system (16%, 46 out of 244) were found to be most common ones (table 1). Overall, men with HF had 55% increased risk of developing cancer compared to the ones without HF (HR 1.55; 95% CI 0.85 –2.80), while women with HF were noticed to have 71% increased risk when compared to the ones without HF after adjustment for BMI, smoking and Charlson comorbidity index (HR 1.71; 95% CI 0.99–2.95).
In 2016 Benke A et al., conducted a prospective trial in Denmark from April 2002 through December 2009 to assess the incidence and risk of cancers among patients with HF. A total of 9307 patients with HF without prior diagnosis of cancer (with LVEF < 45%) were included and a comparison with general Danish population was made. Follow up was done with mean time of 4.5 ± 2.3 years. The primary endpoints of their study were diagnosis of a new cancer and all-cause mortality. Results showed 975 new cases of cancer in HF cohort with an incidence rate of 188.9 [95% confidence interval (CI) 177.2–200.6] per 10 000 patient‐years. They also conducted a subtype-specific Cox regression analysis with age and gender adjustment which demonstrated skin and lung cancers to be most common cancer associations in HF cohort with incidence of 16.3% and 15.7% respectively. Hazard ratios for cancer of kidney, urinary system, liver / biliary, hematologic, digestive, breast are shown in table 1. Risk of breast cancer in women with HF was noted to be increased with HR of 1.36 (95% CI 1.02–1.81, P < 0.038), however risk of prostate cancer in men with HF was not increased (HR:1.04 95% CI, 0.88–1.24, P < 0.6345).
In 2016 Hasin et al., conducted a prospective trial in Minnesota USA from November 2002 through December 2010 to access the incidence and risk of cancer among patients who survived first myocardial infarction (MI). A total of 5,327 patients after first MI were included in study, 228 developed HF (both HFpEF ≥50%, HFrEF with EF < 50%) post MI and 28 out of 228 developed cancer after diagnosis of HF with incidence density rate (per 1,000 person-years) of 33.7 (p = 0.002). Cancer diagnosis was reported in 13% (N =16) of patients with HFrEF and 11% (N =12) with HFpEF. Patients who had HF were found to have more than 2-fold increased risk of cancer (HR: 2.16; 95% CI: 1.39 to 3.35). Most common types of reported cancer were respiratory (29%) and digestive (29%) followed by hematologic (14%).
Table 1: Patient characteristics
|
Author, year, |
Banke, 2016* |
Hasin, 2016 |
Hasin, 2013 |
|
country |
Denmark |
US |
US |
|
(study period) |
(2002-2009) |
(1979–2002) |
(2002–2010) |
|
Type of cancers |
|
|
|
|
All |
975 |
98 |
244 |
|
Digestive tract |
166 |
10-Aug |
48 |
|
Lung |
153 |
12-Aug |
20 |
|
Melanoma |
159 |
8-Feb |
7 |
|
Breast |
47 |
8-Jan |
24 |
|
Kidney/Urinary System |
80 |
4-Jan |
19 |
|
Hematologic |
66 |
5-Apr |
39 |
|
Others |
304 |
15-Feb |
53 |
|
Sample (n) |
4, 968,582 |
1081 |
1192 |
|
HF/non-HF (n) |
9307/4,959,275 |
228/853 |
596/596 |
|
Follow-up (years, mean, SD) |
4.5 ± 2.3 |
4.9 ± 3.0 |
7.7 ± 6.4 |
|
Age (mean, SD) |
67.8 ± 2.2 |
72 ± 14/ 62 ± 15 |
73 ± 14/ 73 ± 14 |
|
Women (%) |
27.4 |
54/37 |
53/54 |
|
Diabetes (%) |
15 |
28/16 |
20/8 |
|
Hypertension(%) |
… |
81/61 |
67/54 |
|
Hyperlipidemia (%) |
… |
66/61 |
29/30 |
|
BMI (kg/m2, mean) |
… |
28.5/29.0 |
27.5/26.5 |
|
Current Smoking (%) |
… |
36/35 |
19/9 |
|
Statins (%) |
49.2 |
81/79 |
… |
|
ACE Inhibitor (%) |
80.9 |
65/58 |
… |
|
NOS Score |
|
|
|
|
*Only data of HF-group is mentioned. |
|||
Discussion
In the present article, we comprehensively reviewed results from 3 cohort studies which demonstrated increased reported risk of all major cancer types in HF patients as compared to non-HF patients.
The intensive hospital encounters, diagnostic evaluations and interventions that occur in HF patients may partly characterize surveillance bias as an explanation for increased risk of cancer. However, sensitivity analysis performed by Banke et al., 2016 and Hasin et al., 2016 excluding individuals who developed cancer within 1 and 5 years of HF diagnosis, respectively, showed persistent association between cancer and HF-cohort. Moreover, most of the increased health care demands of HF patients occur early in the first year after diagnosis[13,14]. Therefore, the median lag time of 1.9 years seen between diagnosis of HF and the diagnosis of cancer; further argues against the assumption that detection bias may enhance or facilitate cancer diagnosis in HF-group.
The increased risk of cancer may relate to shared risk factors between HF and cancer. Diseases like diabetes mellitus, hypertension, metabolic syndrome, dyslipidemia, andobesity are prevalent in HF etiology and individually linked to increased cancer risk[15-18]. In the study by Hasin et al., 2013, the reported risk of cancer was greater in younger patients arguing against a major role of measured and unmeasured comorbidities as their prevalence increases with age.
The association between HF and cancer raises concerns that certain cardiovascular medications may contribute to malignancy. Medications like calcium channel blockers and cardiac glycosides seem unlikely to be involved as none of these agents occupies a central role in treatment of HF and studies have either shown no association with cancer or failed to establish causality[19,20]. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs) and beta-blockers have conflicting role on tumor and vascular angiogenesis, whereas, statins and aspirin demonstrate antitumor properties and are able to reduce cancer risk after long-term use[21,22]. Overall, data on a possible association between cardiovascular medications and cancer have been inconclusive in most cases[22,23]. In our review patients with and without HF did not differ much by statins or angiotensin antagonist’s use. In 2018, FDA recalled valsartan manufactured by Zhejiang Pharmaceuticals for cancer concerns due to detection of trace amounts of N-nitrosodimethylamine (NDMA) which is a known human carcinogen. However, to date no reports of adverse events related to this recall have been received.
While a reason for an increased cancer risk in HF is not apparent from these studies, inflammation may be a possible link. Inflammatory response plays an important role in the tumorigenesis process, and, it is an established component of HF pathophysiology[24,25]. Many studies have established inflammation as a molecular link between these chronic diseases[26]. Hence, it can be speculated that chronic inflammation may be responsible for increased cancer development in HF. Other viable explanations include the possibility that stress from chronic disease or mechanisms, other than inflammation, associated directly with the physiology of HF may also be operating in cancer such as tissue hypoxia and neurohormonal activation[27,28].
As cancer and HF are an enormous health care burden and well-known causes of increased mortality[5,29], our article can have important clinical implications. First, Diagnosis of cancer in HF patients confers excess mortality risk in these patients highlighting the importance of multimorbidity and of vigilant cancer surveillance in the management of HF, since the risk remains increased even after several years. Second, there are various shared risk factors as well as cellular, signaling and genetic pathways common to cardiovascular diseases and cancer, understanding this biological overlap is critical and may help to identify novel therapeutic and preventative strategies for both disorders.
There are several limitations of our review. First, given the observational design of the studies, the observed associations could reflect residual confounding, hence the results should be considered as reflecting an association rather than causality. Second, our study depended only on the data reported in the included studies, some end point data for variables such as all-cause and cause-specific mortality or specific cancer types were unavailable, and considering the limited number of studies with significant heterogeneity, publication and reporting biases were inevitable to some extent. Benke et al., 2016 excluded patients with HFpEF from their trial which might have reduced total reported cases of cancers in HF. Incidence of non-melanomous skin cancers were excluded from both studies conducted by Hasin et al., in 2013 and 2016. Third, due to geographic limitations of the study participants, the generalizability of the results from this study is limited.
Conclusion
In conclusion, the cohort studies in our review indicated that HF is associated with increased incidence of subsequent cancer diagnosis. Further clinical trials are needed to study the common etiology, association of common mechanisms behind their etiology and possible interventions for treatment to reduce poor prognosis.
Acknowledgement
None
Statement of Ethics
Disclosure statement
All authors have no conflict of interest to disclose
Funding Source
None
References
- 1. Mozaffarian, D., Benjamin, E.J., Go, A.S., et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. (2015) Circulation. 131(4): e29-322.
- 2. Thomas, F. L. Heart failure: the epidemic of the new century. (2014) Eur Heart J 35(48): 3389–3390.
- 3. Marco, M., Valerio, Z., Gianfranco, P., et al. Cardiovascular and noncardiovascular comorbidities in patients with chronic heart failure. (2011) J Cardiovasc Med 12(2): 76–84.
- 4. van der Wel, M. C., Jansen, R. W., Bakx, J. C., et al. Non-cardiovascular co-morbidity in elderly patients with heart failure outnumbers cardiovascular co-morbidity. (2014) J Am Coll Cardiol 64(21): 2281-2293.
Pubmed| Crossref| Others
- 5. Lindsey, A., Bray, F., Ferlay, J., et al. Global Cancer Statistics, 2012. (2015) Ca Cancer J Clin 65(2): 87-108.
- 6. Tribouilloy, C., Buiciuc, O., Rusinaru, D., et al. Long-term outcome after a first episode of heart failure. (2010) Int J Cardiol 140(3): 309-314.
- 7. Charlson, M.E., Pompei, P., Ales, K.L., et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. (1987) J Chronic Dis 40: 373-383.
- 8. Banke, A., Schou, M., Videbaek, L., et al. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. (2016) Eur J Heart Fail 18(3): 260-266.
- 9. Hasin, T., Gerber, Y., Weston, S. A., et al. Heart Failure after Myocardial Infarction is Associated With Increased Risk of Cancer. (2016) J Am Coll Cardiol 68(3): 265-271.
- 10. Hasin, T., Gerber, Y., Weston, M. S., et al. Patients with Heart Failure Have an Increased Risk of Incident Cancer. (2013) J Am Coll Cardiol 62(10): 881-886.
- 11. Moher, D., Liberati, A., Tetzlaff, J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. (2010) Int J Surg 8(5): 336-341.
- 12. Wells, G.A., Shea, B., O’Connell, D., et al: The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2013) Ottawa Hospital Research Institute.
Pubmed| Crossref| Others
- 13. Chamberlain AM, Gerber Y, Dunlay SM, et al. Burden and timing of hospitalizations in heart failure: a community study. (2014) Circulation 130.
Pubmed| Crossref| Others
- 14. Dunlay, S.M., Shah, N.D., Shi, Q., et al. Lifetime costs of medical care after heart failure diagnosis. (2011) Circ Cardiovasc Qual Outcomes 4(1): 68-75.
- 15. Berger, S.M., Gislason, G., Moore, L.L., et al. Associations between metabolic disorders and risk of cancer in Danish men and women--a nationwide cohort study. (2016) BMC Cancer 22: 133.
- 16. Wolk, A., Gridley, G., Svensson, M., et al. A prospective study of obesity and cancer risk (Sweden). (2001) Cancer Causes Control 12(1): 13-21.
- 17. Liu, C.S., Hsu, H.S., Li, C. I., et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. (2010) BMC Gastroenterol 10: 51.
- 18. Bui, A. L., Horwich, T. B., Fonarow, G.C. Epidemiology and risk profile of heart failure. (2011) Nat Rev Cardiol 8(1): 30-41.
- 19. Ahr, H. J., Bomhard, E., Mager, H., et al. Calcium channel blockers and cancer: is there preclinical evidence for an association? (1997) Cardiology 88: 68-72.
- 20. Karasneh, R. A., Murray, L. J., Cardwell, C. R. Cardiac glycosides and breast cancer risk: A systematic review and meta-analysis of observational studies. (2017) Int J Cancer 140(5): 1035-1041.
- 21. Ma, C., Wang, Q., Man, Y., et al. Cardiovascular medications in angiogenesis--how to avoid the sting in the tail. (2012) Int J Cancer 131(6): 1249-1259.
- 22. Ocampo, N. V., Tafreshi, J., Hauschild, C. L., et al. Cardiovascular medications and risk of cancer. (2011) Am J Cardiol 108: 1045-1051.
- 23. Alameddine, A. K., Visintainer, P., Normand, S. L., et al. Cancer rates in adults after cardiac interventions: a preliminary observational report. (2014) Am J Clin Oncol.
Pubmed| Crossref| Others
- 24. Fernandes, J.V., Cobucci, R. N., Jatobá, C. A., et al. The role of the mediators of inflammation in cancer development. (2015) Pathol Oncol Res 21(3): 527-534.
- 25. Bouras, G., Giannopoulos, G., Hatzis, G., et al. Inflammation and chronic heart failure: from biomarkers to novel anti-inflammatory therapeutic strategies. (2014) Med Chem 10(7): 682-699.
- 26. Camps, J., García-Heredia, A. Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. (2014) Adv Exp Med Biol 824: 1-4.
- 27. Kocic, B., Filipovic, S., Vrbic, S., et al. Stressful life events and breast cancer risk: a hospital-based case control study. (2015) J BUON 20(2): 487-491.
- 28. Schroten, N. F., van der, P. K., et al. High cumulative incidence of cancer in patients with cardio-renal-anaemia syndrome. (2010) Eur J Heart Fail 12(8): 855-860.
- 29. Stewart, S., Ekman, I., Ekman, T., et al. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004). (2010) Circ Cardiovasc Qual Outcomes 3(6): 573-580.
- Pubmed| Crossref| Others30. Cinciripini, P.M., Wetter, D.W., McClure, J.B. Scheduled reduced smoking: effects on smoking abstinence and potential mechanisms of action. (1997) Addict Behav 22(6): 759-767.
- 31. Hajek, P., Stead, L.F., West, R. Relapse prevention interventions for smoking cessation. (2013) Cochrane Database Syst Rev 20(8).
- 32. Cahill, K., Stevens, S., Perera, R., et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. (2013) Cochrane Database Syst 31(5).
- 33. Snaterse, M., Dobber, J., Minnebo, M., et al. Smoking cessation after an acute coronary syndrome: immediate quitters are successful quitters. (2015) Neth Heart J 23(12): 600-607.