Autologous Adipose Tissue-Derived Mesenchymal Stem Cells Affect the Regeneration of Equine Tendon Lesions
Kathrin Weber1, Sabine Conrad2, Florian Nufer3, Ulrich Walliser1, Thomas Skutella4*
Affiliation
- 1Pferdeklinik Kirchheim, Nürtinger Straße 200, 73230 Kirchheim unter Teck, Germany
- 2P.O. Box 1243, 72072 Tübingen, Germany
- 3Bahnhofstr. 20, 71560 Sulzbach, Germany
- 4Institute for Anatomy and Cell Biology, University of Heidelberg, Im Neuenheimer Feld 307, 69120 Heidelberg, Germany
Corresponding Author
Dr. Thomas Skutella, Professor, Institute for Anatomy and Cell Biology, University of Heidelberg, Im Neuenheimer Feld 307, INF 307, 69120 Heidelberg, Germany, Tel: +49-6221-54-8228; Fax +49-6221-54-5604; E-mail: skutella@ana.uni-heidelberg.de
Citation
Weber K., et al. Autologous Adipose Tissue-Derived Mesenchymal Stem Cells Affect the Regeneration of Equine Tendon Lesions (2016) J Vet Sci Animal Welf 1(1): 1- 10.
Copy rights
© 2016 Weber K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Adipose derived stem cell; Mesenchymal stem cells; Adipose tissue; Tendon lesions; Immunocytochemistry
Abstract
Autologous adipose tissue-derived mesenchymal stem cells (ADSCs) are capable of mesenchymal multi-lineage differentiation including tenocyte-like cells when derived from fat tissue. Clinical data from sports horses with limb superficial digital flexor tendons (SDFT) and suspensory ligament (SL) injuries treated with ADSCs were evaluated in this retrospective study. ADSCs were recovered from subcutaneous tissue of the tail-head. In FACS analysis cultured cells were CD14+/CD105+/CD90+/CD44+ and CD34-/CD45-/CD117- and stained CD90+/CD44+ in immunocytochemistry. After 15 – 30 days of post-diagnosis, we injected ADSCs intralesionally, guided by ultrasound. At regular periods of time an ultrasonography of SDFT and SL injuries and a clinical examination was conducted. In post-treatment, the ultrasonography signal was clearly improved in ADSC-treated SDFT and SL injuries compared to the lesionsite before. The injection of ADSCs did not cause any complications, nor showed any treatment-related adverse events, neither was injection flares substantiated in any of the horses. The healed lesions proved to be stable over a long period of time. For the therapy of sports horses active in different athletic disciplines, acute and chronic pain or fore- and hind-limb SDFT and SL injuries, this effect strongly supports the therapeutic use of regenerative ADSC sculptured from adipose tissue.
Abbreviations :
ADSC: Adipose Derived Stem Cell; MSC: Mesenchymal Stromal Cell; SDFT: Superficial Digital Flexor Tendon; SL: Suspensory Ligament; ESWT: Extracorporeal Shock Wave Therapy; PRP: Platelet Rich Plasma; ACS: Autologous Conditioned Serum; AT: Adipose Tissue; SVF: Stromal Vascular Fraction
Introduction
Commonly occurring musculoskeletal injuries in horses used for athletic contests, dressage horses in particular, are those of the superficial digital flexor tendons (SDFT) and suspensory ligament (SL)[1]. Furthermore, the main problem in the rehabilitation of tendon lesions in sports horses is an inadequate tissue repair of chronic recalcitrant tendon injuries, which often prevents the athlete’s return to the initial performance level[2]. The application of ADSCs could provide a beneficial therapeutic procedure of the treatment for these particular musculoskeletal diseases in horses and humans.
Equine athletes involved in jumping, hunting, or racing activities often suffer from injuries of the SDFT or SL causing disabilities. The equine SL and the topical and superficial and deep digital flexor tendons function as a weight-sustaining and elastic tissue. These ligaments and tendons mainly function in two ways: as support of the metacarpo / tarsophalangeal (fore-limb/hindlimb) joints and as energy storage for springily movements[3,4]. All of these suspensory ligament injuries seem to have vigorous exercise beyond the horse’s physical fitness as a fundamental cause.
It is common for a horse tendon to repair via a process of fibrosis, with a stiffening aftereffect, a severe consequence of performance loss and a risk for re-injury. Instead of healing with ordinary ligamentous tissue, it is typical that ligament healing includes an inflammation followed by the deposition of scar tissue. While the tissue longevity may be restored the affected ligament never recovers its original elasticity, and constricting adhesions are formed[5,6]. Thus, a recovered horse is predestinated for re-injuries and a shortened career.
Hence, the economic impact might be considerable. Cells involved in new tissue synthesis are regarded to be tendon specific progenitor cells, are most probably to be present in the endotenon tissue, between the collagen fascicles and para-vascular[7]. In young tendons, these cells are presented plentifully, whereas mature equine tendons do not seem to have an ample subpopulation of tissue-specific progenitor cells[8].
General therapeutic approaches of tendon lesions include inactivity after the injury and subsequent controlled exercise[9,10], a more aggressive surgical desmoplasty with fasciotomy and the extracorporeal shock wave therapy (ESWT)[10].
The goal of autologous regenerative therapy as an innovative treatment approach is to minimize the risk of scarring, to improve the strength of the affected ligaments, and to render a return to high performance possible for sports horses. One form of autologous regenerative therapies is the platelet rich plasma (PRP) approach, which has been employed for the treatment of suspensory ligament injuries[11]. During PRP treatment a small amount of endogenous growth factors are directly added to the injury site and thus might possibly anticipate the healing process, which allows an earlier come-back to sports after a musculoskeletal injury[12]. Besides, with another blood derived biological, autologous conditioned serum (ACS) or irap an early significant reduction of lameness and temporary improvement of ultrasonographic parameters of repair tissue during equine tendon healing of the SDFT was observed[13]. Equine studies were previously concentrated on the IL-1Ra-mediated anti-inflammatory effects of ACS[13]; yet, the growth factors such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta3 (TGF-β3) in tendon healing should be greater or equally observed[14-16]. Resident precursor cells like mesenchymal stem cells (MSCs) and tenoblasts may possibly be attracted by these growth factors, which tend to augment cell proliferation during tendon healing[17]. Under in vitro conditions 10 - 50 ng/ml IGF1and TGFβ3 are sufficient to proliferate human tendocytes under FBS-free conditions[7,18]. Furthermore, BMP-12 has been shown to induce tendogenic differentiation of MSCs[19]. Table 1
Table 1: Lameness scale.
| Grade degree of lameness (on 5) | |
| 0 | Lameness not perceptible under any circumstances |
| 1 | Lameness is difficult to observe and is not consistently apparent, regardless of circumstances |
| 2 | Lameness is difficult to observe at a walk, or when trotting in a straight line, but consistently apparent under certain circumstances |
| 3 | Lameness is consistently observable at a trot under all circumstances |
| 4 | Lameness is obvious at a walk |
| 5 | Lameness produces minimal weight bearing in motion and/or at rest or a complete inability to move |
Primarily three separate phases merging into each other determine the process of tendon healing. First, phagocytosis and a demarcation of injured tendon tissue constitute the acute inflammatory phase (< 10 – 14 days). Second, the forming of a fibro-proliferative callus proceeds during the proliferative phase (4 – 45 days). Third, the collagen fibrils combine into tendon bundles during the remodeling or maturation phase (45 – 120 days; < 3 months)[20,21].
Another form of autologous regenerative therapy is represented by mesenchymal stem cells (MSCs) derived from bone marrow or, originating from adipose tissue (AT). For decades, this remedy has been employed in veterinary clinical practice for the treatment of ligament and tendon injuries. In both, in vitro and in vivo models, ADSCs proved to be able to differentiate into multiple cell lines including bone, cartilage, tendon, and ligament[22,23]. ADSCs can differentiate into tenocytes in vivo, thus producing increased tensile strength of repaired tendons. These findings imply that their clinical use for tendon injuries is more rational than conventional methods or might be applied in combination with other methods[24].
The results of the treatment of equine experimental tendonitis and naturally occurring tendon and ligament injuries with adipose derived nucleated cells and ADSCs were promising[25]. ADSCs display an expression of extracellular matrix proteins with the highest collagen 1A2 to collagen 3A1 ratios when compared with MSCs from other sources. Furthermore, the second highest expression of the tendon markers tenascin-C and scleraxis revealed by ADSCs[26] implies their particular value for the therapy of equine tendinopathy. Nevertheless, the range of information on the fate of autologous ADSCs after intralesional injection into equine SDFTs is marginal. An equine collagenase tendonitis model[27] lately disclosed the detection of ADSCs labelled with nanocrystals in peripheral blood for 7 days and 72 h post-implantation and of SDFT tissue 7 days after intralesional injection.
Our study by Conze, et al. made the advantages of treatment with autologous ADSCs on neovascularization of artificial equine tendon lesions clear[28]. The findings of our research study exhibited a significantly higher blood flow on the color Doppler sonography two weeks post-treatment, that is, four weeks post-surgery. Additionally a greater number of vessels at post-mortem histology 22 weeks after the intralesional injection of ADSCs into SDFT lesions in contrast to controls were counted. It is wellknown that ADSCs increase the expression of different angiogenic factors like HGF, VEGF, PGF, TGF-beta, Ang-1 and Ang-2[29-31]. Besides, the power Doppler ultrasonography showed an increased blood flow in tendons six weeks after intralesional treatment of collagenase-induced SDFT lesions with ADSCs suspended in platelet concentrate[32]. These study results imply that ADSCs positively affect the therapy of neo-angiogenesis in equine tendon lesions.
In this retrospective study lesions of SDFT and SL were treated with a single application of ADSCs, and the clinical potential of this stem cell therapy was evaluated by clinical examination and ultrasonography.
Materials and Methods
This retrospective study included the evaluation of procedures with four warm blood horses, within the age of 6 - 16 years. In an anterior clinical examination with B-mode ultrasonography and ultrasonographic tissue characterization, a lesion of the SDFT or SL could be diagnosed.
Subcutaneous fat was collected, ADSC isolated and cultured 2 - 4 weeks before the intralesional stem cell application, the horses were sedated and about 1 – 2 cm3 of subcutaneous fat was extracted from the left coccygeal region at the caudate-beginnings. The ADSCs were insulated, brought into cultivation as per ulterior description and accurately defined by the presence or absence of markers.
Description of ADSCs characteristics with FACS and immunocytochemistry For the markers CD34, CD44, CD45, CD90, CD105 and CD117 we completed a FACS analysis. The immunocytochemistry was carried out with the primary antibodies for CD44 and CD90.
Injection of labelled ADSCs Approximately 15 - 30 days post-diagnosis, we attended the horses’ tendon lesions unilaterally with ADSCs. We administered ADSCs under ultrasonographic guidance and utilizing a 23-G, 1-inch needle; roughly 15 × 106 ADSCs suspended in 2 ml 0.9 % saline (total volume 3 ml) and split into aliquots of 1.5 ml were injected into the SDFT or SL lesions. The limbs were dressed with half-limb bandages for 2 days post-cell-implantation.
After treatment
Subsequently the horses were prescribed inactivity for two days followed by a moderate exercise protocol after 3 days.
Magnetic Resonance Imaging (MRI): MRI was conducted with standing low-field MRI: 0.27-Tesla open magnet (Hallmarq Veterinary Imaging, Guildford, Surrey, UK) and a fetlock radiofrequency coil.
Ultrasonography: The ultrasound was performed with Esaote MyLab™ One VET, Mod 8100 ultrasound transducer: SL3323 Vet, 13 – 6 MHz and Sonosite Vet 180 plus Manufactured by: SonoSite, Inc., linear transducer: L52/10-5 (10 MHz). The ultrasound images were scored with 0 to 5. The horses achieved score 1, concurring with initial lesion filling with the presence of hypo-echoic areas a moderate diffuse decrease in echogenicity, lacking of a parallel fiber pattern and 0 – 25% fiber alignment. When a gradual filling of the lesion with the presence of multiple areas with decreased echogenicity was indicated, as well as an increased parallel fiber pattern and 25 – 50% fiber alignment, the horses scored 2. The horses were given score 3, when a less distinct demarcation between injured and uninjured tendon with remaining hypo echoic areas and an increased parallel fiber pattern, 25 – 50% fiber alignment and reduced width and thickness occurred. The horses achieved score 4, hardly indicating any demarcation between injured and healthy tissue, faint hypo-echoic areas, an almost total fiber alignment and very mild increased thickness.
Table 2: Evaluation criteria for the interpretation of ultrasound images
| Score | Echogenicity | Fiber pattern/alignment | Size of ligament |
|---|---|---|---|
| 0 | Anechoic area (central core lesion) | Lacking of parallel pattern acute injury (hemorrhage), 0 - 25% FA | Enlarged width and thickness |
| 1 | Lesion site starting to fill with presence of hypo-echoic areas, and moderate diffuse decrease in echogenicity | Lacking of nice parallel pattern, 0 - 25% FA | Enlarged width and thickness |
| 2 | Lesion site gradually filling with presence of multiple areas with decreased echogenicity | Lacking of nice parallel pattern, 0 - 25% FA | Enlarged width and thickness |
| 3 | Demarcation between injured and uninjured tendon less distinct, hypo-echoic areas are remaining | Increased parallel pattern, 25 - 50% FA | Mild enlarged width and thickness |
| 4 | Hardly any demarcation between injured and healthy tissue, faint signs of hypo-echoic areas | Close to total fiber alignment, 75 - 100% FA | Almost no enlarged width and thickness |
| 5 | Echogenicity (almost) identical to contralateral ligament | Close to total fiber alignment, 75 - 100% FA | No enlarged width and thickness |
Different parameters, such as echogenicity, fiber pattern/alignment (FA) grading and size (width and thickness) of the ligament, were considered and compared to the contralateral limb.
Clinical check-up: Before the stem cell injection, we evaluated the clinical findings additionally after 6 - 16 week and 9 - 24 week-intervals following the stem cell therapy. The same veterinary team evaluated the clinical findings according to the AAEP scoring system.
Results
Cell culture
Within the frame of this study, ADSCs could be generated from adipose tissue samples. The adipose tissue proved to be a plentiful source of stem cells and could be easily retrieved from subcutaneous adipose tissue of the caudate-beginnings. About 10000 - 50000 ADSCs per 1 cm3 adipose tissue could be generated by plastic adherence. After isolating the stem cells, there was an intense enlargement of cells in the culture bottle. After approximately 8 - 10 days under standard MSC cell culture conditions 15 x 106 cells can be produced, i.e. a 330 - 1500-fold enlargement of the original material. Various typical characteristics of MSCs could be verified, like their typical morphological appearance (Figure 1) and the expression/non-expression of important CD surface antigens (see below). At the latest in passage two, an amount of 15 x 106 cells was transplanted. In general, the transplantation was performed 15 - 30 days post-diagnosis. The triline age differentiation potential of the ADSCs was proved according to Dominici, et al.[33]. ADSCs could be differentiated in osteogenic, chondrogenic and adipogenic lineages (data not shown).
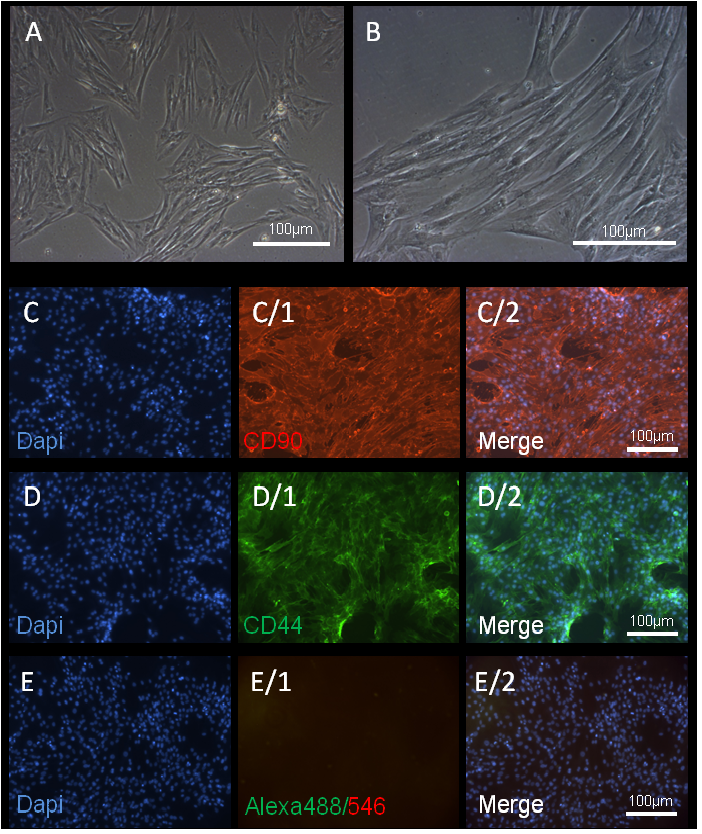
Figure 1: ADSCs cell culture and immunohistochemistry of CD antigens.
(A, B) Example of ADSCs in culture with a typical morphology. These cells show plastic adherent properties under normal culture conditions.
The cultured MSCs also express on their surface CD90 and CD44.
(C) DAPI, (C/1) CD90 immunostaining and (C/2) merged image.
(D) DAPI, (D/1) CD44 immunostaining and (D/2) merged image.
(E-E2) negative control with secondary antibody. Magnification 10-20X and scale bars 100 μm.
FACS and immunohistochemistry
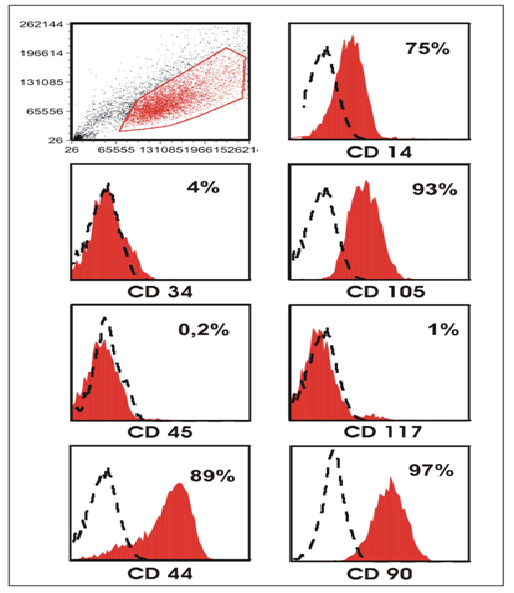
The flow cytometric analysis of cell lines brought typical results for MSCs. They revealed that the surface antigenes CD34, CD45 and CD117 were negative, whereas CD44, CD90 and CD105 were positive. In addition lower levels of CD14 were expressed (Figure 2). Both important surface antigens CD44 and CD90 are displayed here as immunohistologic evidence (Figure 1).
Figure 2: Flow cytometric analysis of cultured ADSCs. Scatter blot and histograms, including percentages, showing the typical profile of CD markers of MSCs.
MRT
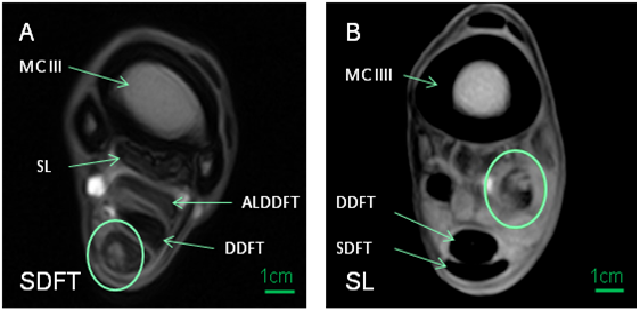
Examples for SDFT and SL lesions are examined by MRI as displayed in (Figure 3).
Figure 3: MRT superficial digital flexor tendons and suspensory ligament lesions
(A) Superficial digital flexor tendon (SDFT) with third metacarpal (MCIII), suspensory ligament (SL), Accessory Ligament of the Deep Digital Flexor Tendon (ALDDFT) and deep digital flexor tendon (DDFT)
(B) SLwith MCIII, DDFT and SDFT
The areas of lesion injury are framed by circles
Clinical assessment
A clinical evaluation was performed according to the AAEP scoring system by the same team of veterinarians. For an adequate evaluation of the entire suspensory apparatus, the ultrasound machine’s imaging controls were sufficiently adapted. For the analysis of clinical data, the horses treated with ADSCs were divided into two patient groups according to the localization of their injury.
In group 1 patients with SDFT lesion were treated which is a quite frequent injury for highly stressed sports horses. Both horses showed a significant lameness of a minor grade, scored 2/5, with a sonographical peripheral defect of the SDFT. Both patients were sound in there-examination after 12 - 16 weeks. In addition, sonographically, all patients showed an improvement of their condition in the form of a deducted defect (Figure 4). No desirable side effects appeared.
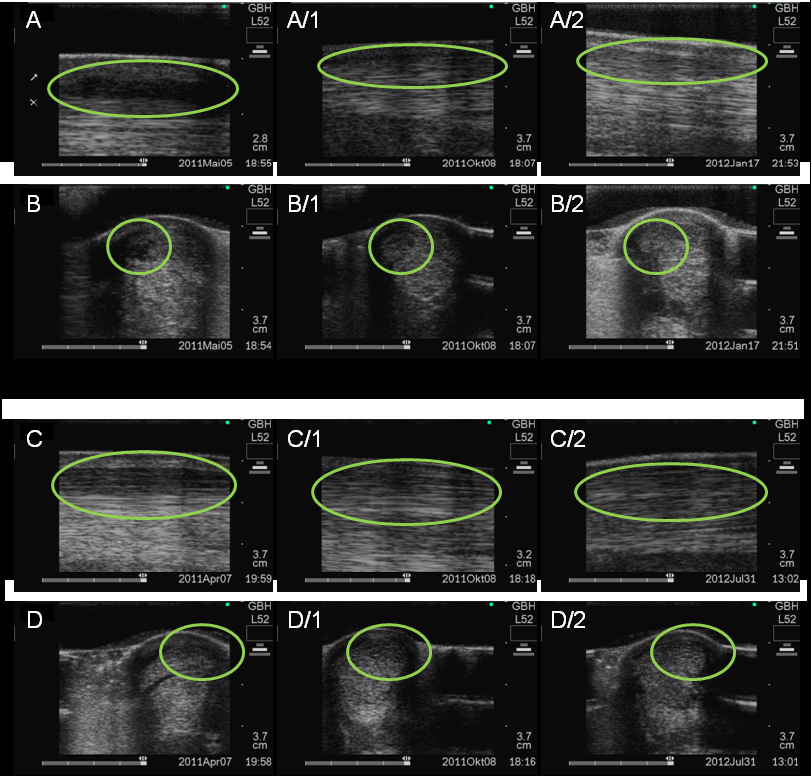
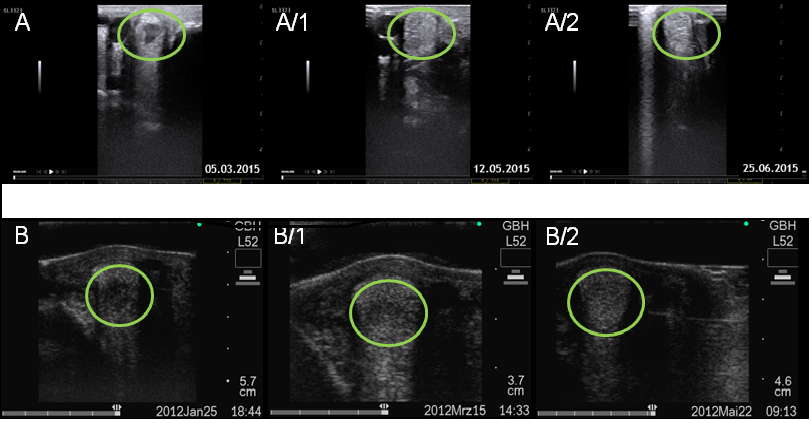
Figure 4: Ultrasound examination of superficial flexor tendon ligament before and after ADSC treatment. Longitudinal (encircled lesion area) and transverse (encircled lesion area) ultrasound images of the superficial digital flexor tendon ligament performed.
In case 1 before stem cell therapywith an anechoic border lesion (A,B) and 5 month after cell transplantation, the area is more echoic (A1-B1) and 7 month after cell transplantation, the area is echoic with a linear fiber alignment (A2-B2).
In case 2 with an hypoechoic area at the palmar border (C,D) and 4 months after cell transplantation, the lesion area is enlarged and diffusely echoic (C1-D1) and 13 months after cell transplantation, the area is more dense but still enlarged (C2-D2).
The areas of lesion injury are framed by circles.
In group 2, two patients were diagnosed with a lesion of the SL branch. Here, both patients could be followed up as well. Both patients exhibited a significant lameness of a minor grade, scored 2/5. The sonographic examination displays in (Figure 5), either a tendinitis or a core lesion of the medial SL. The clinical signs improved after 6 - 14 weeks post stem cell therapy. Both patients showed no clinical signs of lameness in the re-examination. Sonographically, both patients showed an improvement of their condition in the form of a deducted defect (Figure 5). Also in this group no undesirable side effects appeared.
Figure 5: Ultrasound examination of superficial flexor tendon ligament before and after ADSC treatment. Transverse (encircled lesion area) ultrasound images of the superficial flexor tendon ligament performed in case 1 before stem cell therapy (A,B) and 3 month (A1-B1) and 4 month (A2-B2) after cell transplantation and in case 2 (C,D) and 2 month (C1-D1) and 4 month (C2-D2) after cell transplantation
Before Cell therapy a limited remaining hypo-echoic lesion were noticed, whereas this was clearly reduced after 2-3 month, heterogeneous areas with small hypoechoic zonesare observed and the areas became more dense after 3 - 4 month.
The areas of lesion injury are framed by circles.
Discussion
In the described protocol, from 1-2 cm³ of adipose tissue, ADSC cultures could be generated. These MSCs displayed the MSC-typical features in the characterization with FACS-analysis and immunohistochemistry. The performed FACS analyses made evident a presentation of the surfaces antigens CD44, CD105 and CD90. The MSCs showed negative for the surface antigenes CD34, CD45 and CD117. For the most part, these findings correspond with studies of another group[32] and verify dominant cells of mesenchymal lineages in the examined cultures. The evaluation of the administered equine stem cell therapies proved the achievement of excellent results. However, these finding scan only be applied to therapeutic effects and clinical evaluations of lameness during treatment, and to sonographic diagnostics. Concerning the therapeutic effect, lameness was examined during check-up, which had considerably improved in all cases. In all followed-up horses a sonographic improvement of the defect could be observed.
For a more thorough examination of the potential of regenerative, immunomodulatory and inflammatory modulating effects of MSC therapy, it is of great interest to gather profound knowledge about the distribution, kinetics and engraftment of the injected cells in the target tissue[34]. Recently Geburek, et al. (2016)[35] in our study could track autologous ADSCs with in vivo MRI and histology after intralesional treatment of artificial equine tendon lesions. We herein perceived that ADSCs labelled with SPIO particles could be discovered in treated SDFTs during each MRI in T2*- and T1-weighted sequences as far as the end of the 9-week observation phase. All treated tendons included high numbers of SPIO- and GFP-labeled cells, as post-mortem examinations made obvious.
MSCs are thought to have anti-inflammatory effects via their inhibition of T cell mediated responses. To exhaust the potential of MSC therapy in future subsequent studies, this is necessary to be examined. In this context it would be well feasible, to use the anti-inflammatory effect of MSCs in the early phase of inflammation post-lesion for a first cell transplantation and to support regeneration with a second cell transplantation during the proliferation phase. In therapy, the cell transplantations could be well combined with other autologous biological analogs[36-38].
Equine suspensory injuries have been treated with autologous regenerative therapies involving PRP, bone marrow aspirates[39], bone marrow-derived mesenchymal stem cells[40], and adipose derived stem cells (ADSCs)[41]. The endogenous growth factors contained in PRP are supposed to facilitate healing and thus render an early come-back to sports after a musculoskeletal injury[11,12]. After ultrasound-documented suspensory branch desmitis, nine yearling standard-bred race horses included in a clinical trial were treated with PRP, and all of them recuperated and performed at least once during their two-year-old racing season[42]. However, 39 thoroughbred racing horses diagnosed with inflammation of the proximal suspensory branches and sesamoid bones did not show any strong distinctions in the results of saline or PRP treatment groups during any racing year[43].
Adipose tissue provides a plentiful resource for adult stem cells. Even though the stromal vascular fraction (SVF) is variable, it contains approximately 44% of ADSCs, as its principal cell type is the ASC[22]. ADSCs and BM-MSCs show various similar features in growth, morphology, gene expression, and cell surface profiles. It has been demonstrated that ADSCs have immunomodulatory characteristics equal or indeed greater than those of bone marrow-derived stem cells[44-46]. In a collagenase model of superficial digital flexor tendonitis in horses, the distinguished healing process of equine tendons and ligaments with ADSCs was illustrated[47]. The gross and histological morphology of healing tendons was enhanced through intralesional injection of ADSCs, as they increased the cartilage oligomeric matrix protein gene expression significantly in comparison to controls. Obliquely and in terms of mechanism, ADSCs are conducive to tissue healing by generating bioactive proteins including growth factors, chemotactic agents and anti-apoptotic factors[47]. In combination these secreted proteins are capable of stimulating the vascular in growth and recruiting additional adult stem cells for the subsequent stimulation of healing. With the finding that apoptosis is part of the pathogenesis of equine tendonitis, it would also be feasible to block apoptosis (programmed cell death)[48]. As revealed in a retrospective study before, the medical records of cases of equine SL injury following treatment with ADSCs[49] showed the following findings: As acute injuries with n = 6 were only a small amount of all injuries, they all proved a full come-back to prior performance level. In comparison to 73.9 % horses with chronic injuries, this included 88 % of all examined horses.
Moreover, for a come-back to sports the treatment after ultrasound-guided intralesional injection has a better prognosis than conservative therapy approaches, as it supports suspensory ligament healing[50]. The PRP treatment solely did not show any response in the SL injury, and no control group was utilized[51]. Thus, no conclusions concerning the medical necessity of PPR addition for achieving the required clinical effect can be made. Nevertheless, after PRP addition to allogeneic MSCs, a considerable improvement of the clinical results was proven for horses with degenerative joint disease[41]. Stem cell stimulating growth factors present in PRP have probably caused this effect, wherefore the latter substance was added to the tenogenically induced MSCs. To establish more clarity, the effect of PRP on MSC-related therapies for equine tendon healing has to be further studied. Also the impact of repeated injections on the activation the healing process and recovery should be tested.
The application of refined improved scaffolds in the equine patient, including native matrices, synthetic polymers and derivatives of naturally occurring proteins delivering multiple growth factors in a temporal organized pattern, could mimic the extracellular matrix of the real tendon. It would thereby further provide biomechanical support for the transplanted cells by improving cell orientation and facilitating matrix production[52].
In this retrospective study no immunogenic hypersensitivity was anticipated or observed after the stem cell injection, as the reported immune response of autologous MSCs is comparable to that of allogeneic MSCs in equine superficial digital flexor tendons[53].
For a chronic suspensory ligament injury, the administration of a second injection would be justified on the grounds of outstanding hypo-echoic areas and an aberrant fiber pattern. Despite missing reports elucidating the influence of repeated MSC injections on chronic tendon injuries the repetition of MSC treatments of liver fibrosis[53] and cardiomyopathie[54-56] brought significant results in improved tissue repair and functionality compared to single dose treatments. The reason for the noticeable improvements in both organs was the reduced fibrosis, which was accomplished by the remodeling of the collagen network, which also is an essential component in chronic tendon injury[54].
Extensive placebo-controlled field studies are still necessary to give more detailed insight in regenerative capacities and modus operandi of the methods described above. Besides, for a detailed acknowledgement of sustainability of the repaired tissue, it is suggested to gather follow-up data for several years.
Conclusion
In the present pilot study merely a few sport horses were included. For monitoring the effect of the ADSC transplantation over time a staggered approach was used. Thus, the possible effect of inter-individual variations (e.g., age, gender and local infection) on the results cannot be determined based on present findings. The carefully considered injection techniques were supposed to prevent reflux of the cell substrate injected. The amount of tendon-retrieved cells in prospective dose-dependent controlled clinical trials should be quantified, and equine patients should be observed for longer time periods.
The technique of autologous stem cell therapy from adipose tissue offers a variety of possibilities for prompt re-implantation of a large amount of MSCs (up to 1 - 2 x 107 MSCs) into the injured horse tendon. The cells have, especially in combination with tendogenic growth factors (ILK-1, TGFβ3, PDGF, BMP12 and bFGF), the potential to regenerate tendon tissue, instead of less operational scar tissue, which is generated during the conventional treatment of tendon injuries. The multiple signal pathways, directing the tendogenic differentiation of equine MSCs still have to be elucidated in detail. To further the stem cell proliferation and tendogenic differentiation, it would be as well conceivable to apply a combination of growth factors and scaffolds mimicking the aligned natural extra-cellular matrix of the tendon together with cell transplantations.
Competing interests:
The other authors declare that they have no conflicts of interest.
Authors’ contributions:
KW collected the clinical, ultrasonographic data and participated in their analyses and revised the manuscript critically. SC performed culture of the ADSCs, FACS, immunohistochemistry, contributed to the interpretation of the data, and revised the manuscript critically. FN contributed to the FACS and immunohistochemistry data. UW contributed to the coordination of the study and to the interpretation of the results. All authors read, revised and approved the manuscript for publication.
Acknowledgement:
The authors thank Ilse Wagner for corrections of the manuscript. We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Univerity of Heidelberg within the funding program Open Access Publishing.
Abbreviations :
ADSC: Adipose Derived Stem Cell; MSC: Mesenchymal Stromal Cell; SDFT: Superficial Digital Flexor Tendon; SL: Suspensory Ligament; ESWT: Extracorporeal Shock Wave Therapy; PRP: Platelet Rich Plasma; ACS: Autologous Conditioned Serum; AT: Adipose Tissue; SVF: Stromal Vascular Fraction
References
- 1. Murray, R.C., Walters, J.M., Snart, H., et al. Identification of risk factors for lameness in dressage horses. (2010) Vet J 184(1): 27-36.
- 2. Dahlgren, L.A. Review of Treatment Options for Equine Tendon and Ligament Injuries: What’s New and How do They Work? (2005) Proceedings of the Annual Convention—American Association of Equine Practitioners.
- 3. Smith, R.K.W. Physiology of Tendon and Ligament. (2005) Int Vet Inf Service 1903-1905.
- 4. Dyson, S.J., Genovese, R.L. The Suspensory Apparatus. Diagnosis and Management of Lameness in the Horse, 2nd edition (2003) 654-666.
- 5. Patterson-Kane, J.C., Firth, E.C. The pathobiology of exercise-induced superficial digital flexor tendon injury in Thoroughbred racehorses. (2009) Vet J 181(2): 79–89.
- 6. Avella, C.S., Smith, R.K.W. Diagnosis and management of tendon and ligament disorders. (2011) Elsevier Saunders 1157–1179.
- 7. Qiu, Y., Wang, X., Zhang, Y., et al. Development of a refined tenocyte expansion culture technique for tendon tissue engineering. (2014) J Tissue Eng Regen Med 8(12): 955-962.
- 8. Zhou, Z., Akinbiyi, T., Xu, L., et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. (2010) Aging Cell 9(5): 911-915.
- 9. Ross, M.W. Suspensory Desmitis-Management Options. (2006).
- 10. Boening, K.J., Loffeld, S., Weitkamp, K. et al. Radial Extracorporeal Shock Wave Therapy for Chronic Insertion Desmopathy of the Proximal Suspensory Ligament. (2000) AAEP Proceedings 46: 203-207.
- 11. Textor, J. Autologous biologic treatment for equine musculoskeletal injuries: platelet-rich plasma and IL-1Receptor Antagonist Protein. (2011) Vet Clin North Am Equine Pract 27(2): 275-298.
- 12. Bosch, G., Moleman, M., Barneveld, A., et al. The Effect of Platelet-RichPlasma on the Neovascularization of Surgically Created Equine Superficial Digital Flexor Tendon Lesions. (2011) Scand J Med Sci Sports 21(4): 554-561.
- 13. Geburek, F., Lietzau, M., Beineke, A., et al. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. (2015) Stem Cell Res Ther 6:126.
- 14. Dahlgren, L.A., van der Meulen, M.C., Bertram, J.E., et al. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. (2002) J Orthop Res 20(5): 910–919.
- 15. Molloy, T., Wang, Y., Murrell, G. The roles of growth factors in tendon and ligament healing. (2003) Sports Med 33(5): 381–94.
- 16. Heisterbach, P.E., Todorov, A., Fluckiger, R., et al. Effect of BMP-12, TGF-beta1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. (2012) Knee Surg Sports Traumatol Arthrosc 20(10): 1907-1914.
- 17. Dahlgren, L.A., Harvey, S.C. Effect of autologous conditioned serum on the metabolism of normal tendon explants. In: 54th Annual Meeting of the Orthopaedic Research Society; March 2–5. Rosemont, IL, USA: Orthopaedic Research Society; (2008).Poster No. 770.
- 18. Qiu, Y., Wang, X., Zhang, Y., et al. Developmentof a refined tenocyte differentiation culture technique for tendon tissue engineering. (2013) Cells Tissues Organs 197(1): 27-36.
- 19. Gulati, B.R., Kumar, R., Mohanty, N., et al. Bone morphogenetic protein-12 induces tenogenic differentiation of mesenchymal stem cells derived from equine amniotic fluid. (2013) Cells Tissues Organs 198(5): 377-389.
- 20. Patterson-Kane, J.C., Firth, E.C., The pathobiology of exercise-induced superficial digital flexor tendon injury in Thoroughbred racehorses. (2009) Vet J 181(2): 79-89.
- 21. Stashak, T.S., Theoret, C.L., Equine Wound Management. (2008) Wiley-Blackwell 489-508.
- 22. Zuk, P.A., Zhu, M., Ashjian, P. Human Adipose Tissue Is a Source of Multipotent Stem Cells. (2002) Mol Biol Cell 13(12): 4279-4295.
- 23. Park, A., Hogan, M.V., Kesturu, G.S., et al. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. (2010) Tissue Eng Part A 16(9): 2941-2951.
- 24. Uysal, A.C., Mizuno, H. Tendon Regeneration and Repair with Adipose Derived Stem Cells. (2010) Curr Stem Cell Res Ther 5(2): 161-167.
- 25. Nixon, A.J., Dahlgren, L.A., Haupt, J.L., et al. Effect of adipose derived nucleated cell fractions on tendon repair in horses with collagenase-induced tendinitis. (2008) Am J Vet Res 69(7): 928-937.
- 26. Wolfman, N.M., Hattersley, G., Cox, K., et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. (1997) J Clin Invest 100(2): 321-330.
- 27. Carvalho, A.M., Yamada, A.L., Golim, M.A., et al. Evaluation of mesenchymal stem cell migration after equine tendonitis therapy. (2014) Equine Vet J 46(5): 635-638.
- 28. Conze, P., van Schie, H.T., van Weeren, R., et al. Effect of autologous adipose tissue-derived mesenchymal stem cells on neovascularization of artificial equine tendon lesions. (2014) Regen Med 9(6): 743-757.
- 29. Jeon, E.S., Lee, I.H., Heo, S.C., et al. Mesenchymal stem cellsstimulate angiogenesis in a murine xenograft model of A549 human adenocarcinoma through an LPA1 receptor dependent mechanism. (2010) Biochim Biophys Acta 1801(11): 1205-1213.
- 30. Beckermann, B.M., Kallifatidis, G., Groth, A., et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. (2008) Br J Cancer 99(4): 622-631.
- 31. Nakagami, H., Morishita, R., Maeda, K., et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. (2006) J Atheroscler Thromb 13(2): 77-81.
- 32. Carvalho, A.M., Badial, P.R., Alvarez, L.E., et al. Equine tendonitis therapy using mesenchymal stem cells and platelet concentrates: a randomized controlled trial. (2013) Stem Cell Res Ther 4(4): 85.
- 33. Dominici, M., Le Blanc, K., Mueller, I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. (2006) Cytotherapy 8(4): 315-317.
- 34. Lombana, K.G., Goodrich, L.R., Phillips, J.N., et al. An Investigation of Equine Mesenchymal Stem Cell Characteristics from Different Harvest Sites: More Similar Than Not. (2015) Front Vet Sci 2: 67.
- 35. Geburek, F., Mundle, K., Conrad, S., et al. Tracking of autologous adipose tissue-derived mesenchymal stromal cells with in vivo magnetic resonance imaging and histology after intralesional treatment of artificial equine tendon lesions - a pilot study. (2016) Stem Cell Res Ther 7(1): 21.
- 36. Bochev, I., Elmadjian, G., Kyurkchiev, D., et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin Production in Vitro. (2007) Cell Biol Int 32(4): 384-393.
- 37. Tholpady, S.S., Ogle, R.C., Katz, A.J. Adipose stem cells and solid organ transplantation. (2009) Curr Opin Organ Transplant 14(1): 51-55.
- 38. Melief, S.M., Zwaginga, J.J., Fibbe, W.E., et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow counterparts. (2013) Stem Cells Transl Med 2(6): 455-463.
- 39. Herthel, D.J. Enhanced Suspensory Ligament Healing in 100 Horses by Stem Cells and Other Bone Marrow Components. (2001) Proceedings of the Annual Convention of the AAEP 47: 319-321.
- 40. Ferris, D.J., Kisiday, J.D., McIlwraith, C.W., et al. Clinical Follow-Up of Horses Treated with Bone Marrow-Derived Mesenchymal Stem Cells for Musculoskeletal Lesions. (2009) Proceedings of the Annual Convention of the AAEP 55: 59-60.
- 41. Harman, R.J., Cowles, B.C., Orava, J.C. et al. Aetrospective Review of 52 Cases of Suspensory Ligament Injury in Sport Horses Treated with Adipose-Derived Stem and Regenerative Cell Therapy. (2007) Proceedings of Veterinary Orthopedic Society 34: 28.
- 42. Waselau, M., Sutter, W.W., Genovese, R.L., et al. Intralesional Injection of Platelet-Rich Plasma Followed by Controlled Exercise for Treatment of Midbody Suspensory Ligament Desmitis in Standardbred Racehorses. (2008) J Am Vet Med Assoc 232(10): 1515-1520.
- 43. Garrett, K.S., Bramlage, L.R., Spike-Pierce, D.L., et al. Injection of Platelet- and Leukocyte-Rich Plasma at the Junction of the Proximal Sesamoid Bone and the Suspensory ligament Branch for Treatment of Yearling Thoroughbreds with Proximal Sesamoid Bone Inflammation and Associated Suspensory Ligament Branch Desmitis. (2013) J Am Vet Med Assoc 243(1): 120-125.
- 44. Vidal, M.A., Kilroy, G.E., Lopez, M.J., et al. Characterization of Equine Adipose Tissue-Derived Stromal Cells: Adipogenic and Osteogeniccapacity and Comparison with One Marrow-Derived Mesenchymal Stromal Cells. (2007) Vet Surg 36(7): 613-622.
- 45. Bochev, I., Elmadjian, G., Kyurkchiev, D., et al. Mesenchymal Stem Cells from Human Bone Marrow or Adiposetissue Differently Modulate Mitogen-Stimulated B-Cell immunoglobulin Production in Vitro. (2008) Cell Biol Int 32(4): 384-393.
- 46. Melief, S.M., Zwaginga, J.J., Fibbe, W.E., et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. (2013) Stem Cells Transl Med 2(6): 455-463.
- 47. Nixon, A.J., Dahlgren, L.A., Haupt, J.L., et al. Effect of Adipose Derived Nucleated Cell Fractions on Tendon Repair in Horse with Collagenase-Induced Tendinitis. (2008) Am J Vet Res 69(7): 928-937.
- 48. Hosaka, Y., Teraoka, H., Yamamoto, E., et al. Mechanism of cell death in inflamed superficial digital flexor tendon in the horse. (2005) J Comp Pathol 132(1): 51-58.
- 49. Herthel, D.J. Enhanced Suspensory Ligament Healing in 100 Horses by Stem Cells and Other Bone Marrow Components. (2001) Proceedings of the Annual Convention of the AAEP 47: 319-321.
- 50. Ferris, D.J., Kisiday, J.D., McIlwraith, C.W., et al. Clinical Follow-Up of Horses Treated with Bone Marrow-Derived Mesenchymal Stem Cells for Musculoskeletal Lesions. (2009) Proceedings of the Annual Convention of the AAEP 55: 59-60.
- 51. Guest, D.J., Smith, M.R., Allen, W.R. Monitoring the fate of autologous and allogeneic mesenchymal progenitor cells injected into the superficial digital flexor tendon of horses: preliminary study. (2008) Equine Vet J 40(2): 178–181.
- 52. Yang, G., Rothrauff, B.B., Tuan, R.S. Tendon and ligament regeneration and repair:clinical relevance and developmental paradigm. (2013) Birth Defects Res C Embryo Today 99(3): 203-222.
- 53. Miryounesi, M., Piryaei, A., Pournasr, B., et al. Repeated versus single transplantation of mesenchymal stem cells in carbon tetrachloride-induced liver injury in mice. (2013) Cell Biol Int 37(4): 340–347.
- 54. Spaas, J.H., Guest, D.J., Van de Walle, G.R. Tendon regeneration in human and equine athletes. (2012) Sports Med 42(10): 871–890.
- 55. Richardson, J.D., Psaltis, P.J., Frost, L., et al. Incremental benefits of repeated mesenchymal stromal cell administration compared with solitary intervention after myocardial infarction. (2014) Cytotherapy 16(4): 460–470.
- 56. Yu, Q., Li, Q., Na, R., et al. Impact of repeated intravenous bone marrow mesenchymal stem cells infusion on myocardial collagen network remodelling in a rat model of doxorubicin-induced dilated cardiomyopathy. (2014) Mol Cell Biochem 387(1-2): 279–285.