Cerebellum and endocannabinoid receptors: a new neurobiological link for mitragynine (Mitragyna speciosa Korth) abuse liability
Affiliation
- 1Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia
- 2Centre for Neurosciences Service and Research, Universiti Sains Malaysia
- 3Centre for Drug Research, Universiti Sains Malaysia
- 4Department of Psychiatry and Psychotherapy, Friedrich-Alexander-University Erlangen-Nuremberg, Germany
Corresponding Author
Muzaimi Mustapha, Department of Neurosciences, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian Kelantan, Malaysia. Tel: +6097676309; Fax: +6097673833; E-mail: mmuzaimi@usm.my
Citation
Nanthini, J., et al. Cerebellum and Endocannabinoid Receptors: A New Possible Neurobiological Link for Mitragynine (Mitragyna Speciosa Korth) Abuse Liability. (2015) J Addict Depend 1(1): 1-7.
Copy rights
© 2015 Nanthini, J. This is an Open access article distributed under the terms of creative Commons Attribution 4.0 International License.
Keywords
Cerebellum; Endocannabinoid; CB1 receptor; Mitragyna speciosa; Mitragynine; Addictive behaviour
Abstract
Drug abuse is a major concern worldwide and has remained so for decades. Beside synthetic drugs, the misuse of psychoactive compounds from natural products as drugs of abuse contributes to this incessant concern. Reports on misuse of mitragynine, an alkaloid from the plant Mitragyna speciosa Korth (Kratom or Ketum) as a substituent for opioid withdrawal syndrome are becoming prevalent in recent years, and extended beyond the geographical boundaries of the plant. Although there are comprehensive data available on chemical and pharmacological properties of mitragynine particularly on its opioid-like analgesic property, the mechanism of actions for its abuse liabilities remain unclear. This article presents the plausible leads for mitragynine abuse liability by reviewing the established roles of endocannabinoid system as a molecular target where the opioids and cannabinoids act. These leads build for the arguments to posit the involvement of ubiquitous endocannabinoid receptors within the cerebellum and the evidences for cerebellar non-motor functions (brain reward processes, drug-induced long term memory and plasticity, and structural alterations linked to addiction) towards a potential new player as the basis for abuse liability of Kratom, accumulating to addiction.
Introduction
Global misuse of psychoactive phytochemical mitragynine from Mitragyna speciosa is an emerging trend. Mitragyna speciosa is a rubiaceous herbal tree indigenous to swamp areas in the Southeast Asia, notably Malaysia and Thailand[1,2]. Known to the locals as “Ketum or Biak-Biak” among the Malaysians, or “Kratom” ‘Kakuam’, ‘Ithang’ or ‘Thom’ in Thailand[3], the plant has been traditionally used as a regional folk remedy. First described and named by Dutch botanist Pieter Willem Korthals, this plant has clustered flowers that are yellow and each of the flower encompassed approximately 120 florets[2]. The active part of Ketum is their dark green leaves which are oval in shape with a slender end (ovate-acuminate) and are able to grow over 7 inches in long and 4 inches wide[2]. Since time immemorial, the leaves of this plant have been used for ailments such as fever, cough, pain and diarrhoea, to deworm, malaria, to improve blood circulation and also even to treat diabetes and hypertension[4,5]. Besides, Ketum is also popular among the open-field laborers who use Ketum for heightened work endurance under the hot sun[2]. In addition, the use of Ketum as a potential psychoactive dietary supplement is evident from anecdotal reports as early as the 19th century[2]. In recent times Ketum is being utilised for opium substitute and weaning addicts off morphine and has since liable to addictive and misuse potentials.
In this paper, we elaborate on two potent alkaloids of Ketum and their known opioid-like effects. We recapitulate the evidences and findings on reciprocal interaction between opioids and endocannabinoids, and posit the possible neurobiological link in exerting the addictive potential of mitragynine. Building from this basis, we extend the current understanding on the role of cerebellum in higher cognitive functions through the involvement of abundant cerebellar endocannabinoid CB1R, particularly in regulating the reward and drug-seeking behavior. The objective of this review, therefore, is to propose plausible leads involving cerebellar endocannabinoid CB1R and mitragynine, as the potential new basis for mitragynine/Ketum abuse liabilities.
The Active Constituents of Mitragyna speciosa
About 40 compounds have been extracted from the leaves of Ketum and the mitragynine is the most active, primary constituent of this plant[6]. Apart from mitragynine, other alkaloids of Ketum previously reported include speciogynine, speciociliatine, paynantheine and 7-hydroxymitragynine[7]. The 7-hydroxymitragynine is a more potent but less abundant congener of mitragynine[2,7]. It is also recognised that the presence of different types of alkaloids and their percentage varies based on their geographical region[7,8]. For instance, the total amount of mitragynine derived from Ketum of Thai origin accounts for about 66% while about 12% of mitragynine from Ketum of Malaysia origin[7]. The chemical structure of mitragynine had been fully determined by Zacharias using the X-ray crystallography in 1965[9]. The identification of lowest energy conformation through computational study further validated the X-ray crystal structure geometry of this alkaloid[10]. The chemical structure of this indole alkaloid appeared to be relate to the structure of yohimbine and voacangine[2].
Pharmacology of Mitragynine and 7-Hydroxymitragynine
While Ketum has many valuable therapeutic claims since the olden days, mitragynine has been identified to be involved and responsible for the unique properties of this medicinal plant. The effects and modes of actions of the alkaloid are very much morphine-like. However, as mitragynine differs structurally from morphine, the spectrum of pharmacological properties of mitragynine also differ[7]. The Methoxy- (MeO) functional group which is present at the ninth carbon of the mitragynine structure is responsible for its anti-nociceptive property[6]. Mitragynine prolonged the latency of nociceptive responses in hot plate, tail pinch test and inhibition of writhing responses[7,11,12]. However, the minor alkaloid, 7-hydroxymitragynine also exhibited anti-nociceptive activities and showed 13 and 46 folds higher activity than morphine and mitragynine respectively[7,13]. The analgesic effects of both mitragynine and 7-hydroxy mitragynine primarily involve the activation of the opioid receptors. The binding assay studies have shown that mitragynine has a relatively highest affinity for μ-opioid receptors followed by κ- and δ- opioid receptors[14,15] and in comparison to morphine, mitragynine has a higher affinity towards δ- and κ- opioid receptors[2]. A detailed study on the involvement of specific opioid receptor subtype showed unlike morphine, mitragynine exerted its anti-nociceptive property through both μ- and δ- opioid receptors[16]. The mitragynine inhibited the contraction of electrically stimulated guinea pig ileum[17] and mouse vas deferens[18] through opioid receptors in a dose-dependent manner. Despite these evidences for the interaction between mitragynine and the opioid receptors, mitragynine is also known to exert its physiological effects through various systems. The central monoaminergic system was shown to be involved in the pain modulation activity of mitragynine. The noxious stimulus test showed the descending noradrenergic and serotonergic systems modulates the anti-nociceptive activity of mitragynine on tail flick test (mechanical stimulation) while the descending noradrenergic system regulates the anti-nociceptive activity of mitragynine hot-plate test (thermal stimulation)[19]. The mitragynine suppression of head twitch response caused by 5-methoxy-N, N-dimethyltryptamine demonstrated the effect of mitragynine on serotonergic function in the brain[20]. Meanwhile, 7-hydroxymitragynine also exerted its effects through the opioid receptors, mainly the μ- receptors[13,21]. In a tail-flick test, 7-hydroxymitragynine induced anti-nociceptive effects through the μ- receptors in mice[22]. The administration of 7-hydroxymitragynine also led to full substitution to the morphine’s discriminate stimulus. This effect can be reversed by treatment with naloxone which demonstrated the involvement of opioid receptors[23].
The Dose-Dependent Effects of Mitragynine
The effects of mitragynine changes depending upon the level of exposure, i.e. it is dose-dependent. The dual effects of mitragynine had been demonstrated in both rodent and human studies where at lower doses, it gave coca-like ecstatic effects while at higher doses it gave opioid-like depressant effects[3,23]. The administration of mitragynine also showed dose-dependent anti-nociceptive response in acetic acid-induced writhing test and formalin test[24]. The mitragynine inhibited the contraction of electrically stimulated guinea pig ileum[17] and mouse vas deferens[18] through opioid receptors in a dose-dependent manner. The mitragynine administration also showed significant dose-dependent increase in latency time in the hot plate test in mice[25,26]. Besides pain relieving property, the mitragynine has been found to have other various physiological effects in dose-dependent manner both in-vitro and in-vivo studies. A dose-dependent manner of inhibition of oxygenase followed by suppression of inflammatory mediator in RAW264.7 macrophage cells suggested the possible anti-inflammatory properties of mitragynine[27]. In a rodents study using anaesthetised rats, the dose-dependent inhibitory effects of mitragynine on 2-deoxy-D-glucose-stimulated gastric acid secretion also being confirmed[28]. Limited studies also demonstrated the effect of mitragynine in cognitive function. In mice, 28 days of chronic administration of mitragynine (5, 10 and 15 mg/kg) significantly impaired the working memory and reduced movement activity in open field test[4]. Mitragynine had a significant dose-dependent effect on locomotor activity where at low dose it resulted in hyperlocomotion and at high dose, hypolocomotion[3]. The same study observed dose-dependent anxiolytic effects and Conditioned Place Preference (CPP) which reflected the rewarding properties of mitragynine in mice[3].
An increase in the horizontal and vertical exploratory activity in the Y-maze was observed following acute oral exposure of mitragynine (20, 40 and 80 mg/kg) in Swiss albino mice[29]. A recent study on the discriminative stimulus properties of mitragynine in rats showed that the mitragynine dose dependently substituted fully to the morphine discriminative stimulus[23]. Although this plant and its derivatives are known for their unique properties for posing both opioid- and psychostimulant- like drug effects, the exact central system and pathways which are involved in modulating the addictive reward process of mitragynine remains elusive.
The Endocannabinoid System
The Endocannabinoid (ECB) is a signaling system which consists of ECB ligands, receptors and enzymes which are responsible for their synthesis, hydrolysis and reuptakemechanism. The ligands of ECB system are anandamide, 2-arachidonic and 2-arachidonyl glycerol[30,31]. There are two subtypes of CBR, CB1R and CB2R. Although distributed throughout the body, the CB1R can be found more predominantly in the brain while the CB2R is more prominently found in the immune cells[32,33]. The CB1R is seven trans membrane domain G-protein coupled receptor, thus the G protein activation mediates the signal transduction. Although the meso corticolimbic dopamine system is globally known for its pivotal role in reinforcing effects of drugs of abuse, the ECB system also plays a significant part in drug-seeking reinforced by drug-related cues and drug-relapse. The CB1R is well known for its role in mediating the effects of the psychoactive compounds[32]. In CBR1 knock-out mice, studies had the demonstrated the mice failure to acquire self-administration and CPP, as well as less withdrawal syndrome to morphine, nicotine, cocaine, alcohol, psycho stimulants and Δ-9-Tetrahydrocannabinoid (THC)[34,35]. Thus, given the similar properties between mitragynine and morphine and the known crosstalk between opioids and ECBs, it is plausible to postulate that the cannabinoid receptors may also be involved in mitragynine drug-seeking and reinforcement motivational behaviour within the brain reward pathways
The Functional Interaction of Endogenous Cannabinoid and Opioid Systems
Opiates and cannabis are two naturally occurring psychotropic drugs that are in use in human culture since the ancienttimes. Morphine which is being used as major analgesic, interacts with the ubiquitously distributed seven transmembrane G-protein coupled opioid receptors[36]. On the other hand, the psychoactive effects of THC are centrally mediated by activation of the cannabinoid receptor of the ECB system. In addition to opioid system, the ECB system has been shown to involve in exerting the anti-nociceptive features of morphine and vice versa. Besides the anti-nociceptive effects, the reciprocal interaction between the opioid-ECB system also exists in reinforcement effects[37-39]. Although the mitragynine is claimed to be opioid-like, however, the recent study on the involvement of ECB system in the anti-nociceptive property of acute mitragynine administration showed no participation of cannabinoid receptors[26]. Nevertheless, the participation of the ECB in reinforcement and addictive effects of mitragynine is unknown.
Cerebellum Functions: Beyond Motor Control
Over several decades cerebellum is recognized as the maestro of motor control. The manifestation of motor in coordination of voluntary movement (termed ataxia) is linked to cerebellar lesions[40]. However, the notions on involvement of cerebellum in cognitive functions started to revolve and were being proposed by researchers two decades ago, particularly based from the findings of neuroimaging studies. The Positron Emission Topography (PET) study demonstrated the activation of cerebellum in relation to ejaculation[41]. Further detailed study on this showed ejaculation related stimulation of the cerebellar dentate nucleus and anterior vermis[42]. In a different study, the observation of an increased mid-vermis granule neurons activation in relation to sexual provocation and ejaculation in rats hinted the involvement of cerebellum in heightened emotional responses[43]. Besides cerebellum involvement in semantic discrimination and sexual behavior, its role in learning and memory has also been implicated. A human PET imaging study conducted to correlate the brain region activation in relation to spatial memory and object recognition revealed activation of the cerebellum with regards to long term memory retrieval[44]. The cerebellum has been implicated in fear memories. Furthermore, the role of cerebellum for perceptual events has been studied where fMRI imaging of the brain during a temporal-spatial task showed the involvement of lobule VII Crus I which implied cerebellar involvement in perceptual predictions[45].
Implicating Cerebellum in Addiction
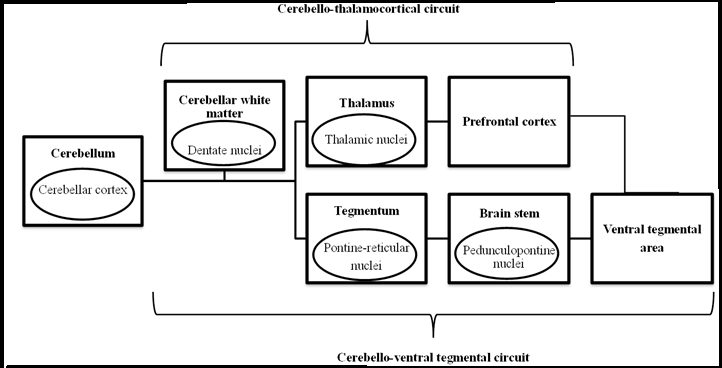
In addition to executive, non-motor functions, evidences are accumulating for the role of cerebellum in development of drug addictive behavior[40]. The basis to examine cerebellum and its role in cognitive functions and drug dependence can be tackled from the structural and functional connections the cerebellum has with various brain regions, such as the memory andbrain rewards circuitry. The recorded neuronal responses in the amygdala and hippocampus following electrical stimulation of the cerebellar fastigial nucleus with relatively short delay times showed direct connections between the areas and cerebellum[46]. Furthermore, no delay was observed in the cerebellar vermis response time in fastigial nucleus in the lesioned cat[46]. In addition, human studies using the Transcranial Magnetic Stimulation (TMS) with stimulation across cerebellar vermis has resulted in an increased theta activity in the Pre-Frontal Cortex (PFC)[47]. Findings of such studies further supported the earlier described cerebellar modulation of various cognitive, non-motor functions through functional pathways between cerebellum and other brain structures. Regarding the participation of cerebellumin addiction and relapse, emerging body of evidences suggested the fronto-cerebellar circuitry (Figure 1.1) to implicate cerebellum in the drug-seeking motivated behavior. The PFC is well known for its involvement in executive controls such as decision making and emotion. The PFC is highly associated to the brain’s reward circuit, the limbic system. The cerebellum, on the other hand, is interconnected to the PFC through the reciprocal connection between Brodmann area 9 and 46 of PFC and cerebellum via dentate nuclei which project through thalamus while the PFC sends feedback via the pontine nuclei[48]. The Brodmann area 9 which is also known as the dorsal PFC acts as one of the important component in regulating the reward process.
Figure 1: The schematic illustration of the erebello-thalamocortical and cerebello-ventral tegmental circuit pathway. In both circuits, the projections originate from the cerebellar cortex which projects into the dentate nuclei of cerebellar white matter. In the cerebello-thalamocortical circuit, the projection from dentate nuclei will projects to the thalamic nuclei of thalamus and terminates in the prefrontal cortex. In cerebello-ventral tegmental circuit, the projection from dentate nuclei will project into the pontine-reticular nuclei of tegmentum that in turn projects into ventral tegmental area via pedunculopontine of brain stem. The VTA dopaminergic cell bodies finally terminate in the PFC
Moreover, the cerebellar projections also projects to the mesolimbic Ventral Tegmental Area (VTA) via cerebellar dentate nuclei which pass thorough the reticulo-tegmental nuclei that project to pedunculopontine nuclei. The VTA dopaminergic cell bodies finally terminate in the PFC (Figure 1.1)[49-52]. Taken together, these anatomical connections suggest the reciprocal interactionbetween the PFC and the cerebellum in cognition regulation as well as the possible dopamine release in mesolimbic VTA and in PFC activated by the cerebellar projection.
The relevance of the PFC and cerebellum working in parallel has been shown through data from several studies. The alterations or diminished fronto-cerebellar circuitry in relation to drug usage has been observed. The fMRI study on working memory of alcoholics showed greater activation of the frontal together with the activation of cerebellar regions in order to achieve the equal performance level as compared with controls[53]. Similarly, the study on the working memory of cocaine users showed cerebellar hyperactivity to compensate the hypoactivity of cingulate cortex[54]. Analysis of alcohol dependents showed activation of dorsolateral PFC-cerebellar VIII system during resting state while the dorsolateral PFC-cerebellar VI system were activated during task engaged. The observed results were in parallel to normal subjects and thus added evidence for the recruitment of cerebellar-based functional networks as a compensatory act by the brain for normal functionality[55]. The study on subordinate hand finger tapping task in alcohol abstainers correlated with the deficits in functionality of pre motor Brodmann area 6 and cerebellar Lobule VI as well as prefrontal Brodmann area 9 and cerebellar Lobule VIII[56]. The fMRI study on the neural activity and functional reorganisation in heroin dependent subjects showed decreased amplitude of low frequency fluctuation in prefrontal regions accompanied by increase in cerebellar region[57]. Also, the cerebellum encodes an internal model that reproduces the properties of PFC. Therefore, in the event of decrease in the functionality of PFC the cerebellum which has previously stored the information from PFC will be activated to represent the PFC[58]. Collectively, these findings imply cerebellar activation in the event of reduced prefrontal control activity. These anatomico-functional arguments lend cohesive argumentsin extending the involvement of cerebellum in reward paradigm and modulation of drug-seeking behaviour.
The plausible leads for the involvement of cerebellum in drug seeking behaviour can be seen from the dopaminergicneurons in the cerebellum. The observation of a decreased in the level of cerebellar dopamine following lesions in the midbrain dopaminergic regions, proved that the dopaminergic cell bodies projected from the retrorubal-substania nigra-VTA to the cerebellum[59]. The presence of dopaminergic A10 group in both primates[60] and rodents[61] has been identified. The projection of A10 cell group from VTA into rat cerebellar cortex terminates at the GL and PL[61]. In an immune cytochemical study, the widespread innervations of dopamine fibres in the rat cerebellum reported presence of intense amount of dopamine afferent fibres in the ML[62]. The study also demonstrated the presence of dopamine membrane transporters in the cerebellar vermis, granule cell layers[63]. The study on the human cerebellum revealed the presence of mRNA for Dopamine Transporter (DAT) in the structure[64]. While the cerebellum receives projections from the VTA, the VTA itself receives cathecolamine fibres[65] and fibre from lateral and interpositus cerebellar nuclei[61].
Besides these, analogous to the limbic circuit dopamine, the cerebellar dopamine system has also been observed toparticipate in drug related activities. Klitenick and co-workers showed increased c-fos expression in rat cerebellum following administration of cocaine and dextro amphetamine. The expression was eradicated by SCH233390, D1 receptor antagonist[66]. In addition, an upregulation of cerebellar D2-like receptors was detected following inoculation of dextro amphetamine in rats[67]. Cocaine has been reported to sensitise c-fos mRNA in the cerebellum with the aid of dopamine D1 and D2 receptors[68]. Together, these results suggest that the cerebellum is likely to be involved in development of addictive behaviour.
Moreover, in relation to the endocannabinoid system, the CB1R that are widely spread in brain with a vast expressionin the cerebellum[69,70]. The CB1R are highly expressed in ML, Climbing Fibre (CF), Parallel Fibre (PF) and BC especially at the BC-PC synapse[71]. Meanwhile, in younger animals, the presence of CB1R has been detected in axons and synaptic terminals of cerebellar BC, SC and PF and CF[72]. The CB1R are expressed predominantly at presynaptic of the neurons that synapse onto PC. The Long Term Depression (LDP) and Long Term Potentiation (LTP) represent the key candidate mechanisms for learning and memory processes associated with addiction[73,74]. The cerebellar CB1R in PF regulates short term plasticity and LTD[75]. Thus, the CB1R induced cerebellar synaptic plasticity may causatively contribute to drug addictive behaviour.
The role of CB1 in modulating the dopaminergic system is known as one of the mechanisms in drug addiction. The inhibition of Gamma-Aminobutyric Acid (GABA) release will cause an increase in the dopamine release[76-78]. In cerebellum, the CB1R are localized in presynaptic GABAergic neurons. The signaling of these presynaptically expressed CB1R could depress the GABA release which in turn increases the dopamine level. Thus, within the cerebellum, the activation of presynaptically located CB1R possibly depresses GABA release and eventually disinhibits the dopaminergic system. This shows a predominant role of cerebellar CB1R in inactivating the GABAergic pathways and contributes to the development of addictive behaviour.
The role of ECB system as retrograde messenger candidates in cerebellum has been demonstrated. In a study, the activation of CF of Naval Medical Research Institute (NMRI) mice brain slices triggers the production of ECBs. The post synaptically produced ECBs bind to presynaptic CB1R which in turn inhibit the GABAergic input to PC. The reversal of this effect by CB1R antagonist, rimonabant further validates the roleof CB1R as retrograde messenger in cerebellum[79]. Overall, the CB1R mediated cerebellar plasticity and fine-tuned regulation of neural transmission may also contribute to the development ofdrug addiction, dependence and relapse.
Conclusion
The emerging new psychoactive substances, in particular the phytochemical of plant mitragynine from Mitragyna speciosa (Ketum) has become a global concern. Mitragynine’s potential as a psychoactive plant appeared anecdotally as an opium substitute and has since emerged with an abuse liability. Targets within the brain-reward circuitry are implicated in drug-induced synaptic plasticity of addiction. The involvement of ECB system in positive modulatory effects triggering the addiction and relapse has been assessed. Also, the ECB system represents a novel class of signalling molecules that contribute to short- and long-term synaptic plasticity throughout the brain through the interaction of CB1R. The interaction between opioids and ECB system in reinforcing effects of addictive drugs and in modulating the synaptic plasticity has been elaborated.
Although the meso corticolimbic dopaminergic region has always been recognised to be central to reward processing,recent evidences implicate the involvement of the cerebellum in cognitive functions with an emerging role in the transition from a pattern of recreational drug taking to the development of an addictive pattern of behavior. The neuroplasticity changes in the cerebellum have been shown to be mediated by endocannabinoid-dependent cellular mechanisms. The ubiquitous CB1R in cerebellum could be the main signaling molecule that may mediate the neuroplasticity changes in the cerebellum contributingfor the involvement of the cerebellum in drug-induced conditioned memory. Thus, given mitragynine’s opioid agonism, it is postulated that the CB1R within the cerebellum may well represent the novel neurochemical basis of mitragynine/Ketum abuse liabilities. Further research is warranted to ascertain the role of CB1R as potential candidate within the cerebellum and its connection with the brain rewards circuitry.
References
- 1. Gong, F., H-p, Gu., Q-t, Xu., et al. Genus Mitragyna: Ethnomedicinal uses and pharmacological studies. (2012) Phytopharmacology 3(2): 263-272.
- 2. Hassan, Z., Muzaimi, M., Navaratnam, V., et al. From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. (2013) Neurosci Biobehav Rev 37(2): 138-151.
- 3. Yusoff , N.H., Suhaimi, F.W., Vadivelu, R.K., et al. Abuse potential and adverse cognitive effects of mitragynine (kratom). (2014) Addiction biology.
- 4. Chittrakarn, S., Sawangjaroen, K., Prasettho, S., et al. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. (2008) J Ethnopharmacol 116(1): 173-178.
- 5. Apryani, E., Hidayat, M.T., Moklas, M.A., et al. Effects of mitragynine from Mitragyna speciosa Korth leaves on working memory. (2010) J Ethnopharmacol. 129(3): 357-360.
- 6. Stolt, A.C., Schroder, H., Neurath, H., et al. Behavioral and neurochemical characterization of kratom (Mitragyna speciosa) extract. (2014) Psychopharmacology 231(1): 13-25.
- 7. Adkins, J.E., Boyer, E.W., McCurdy, C.R. Mitragyna speciosa, a psychoactive tree from Southeast Asia with opioid activity. (2011) Curr Top Med chem 11(9): 1165-1175.
- 8. Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. (2004) Chem Pharm Bull 52(8): 916-928.
- 9. Babu, K.M., McCurdy, C.R., Boyer, E.W. Opioid receptors and legal highs: Salvia divinorum and Kratom. (2008) Clin Toxicol (Phila) 46(2): 146-152.
- 10. Zacharias, D., Rosenstein, R., Jeffrey, G. The structure of mitragynine hydroiodide. (1965) Acta Crystallographica 18(6): 1039-1043.
- 11. Liu, H., McCurdy, C.R., Doerksen, R.J. Computational Study on the Conformations of Mitragynine and Mitragynaline. (2010) Theochem 945(1-3): 57-63.
- 12. Reanmongkol, W., Keawpradub, N., Sawangjaroen, K. Effects of the extracts from Mitragyna speciosa Korth. leaves on analgesic and behavioral activities in experimental animals. (2007) Songklanakarin J Sci Technol 29(Suppl 1): 39-48.
- 13. Sabetghadam, A., Ramanathan, S., Mansor, S.M. The evaluation of antinociceptive activity of alkaloid, methanolic, and aqueous extracts of Malaysian Mitragyna speciosa Korth leaves in rats. (2010) Pharmacognosy Res 2(3): 181-185.
- 14. Matsumoto, K., Horie, S., Ishikawa, H., et al. Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. (2004) Life sciences 74(17): 2143-2155.
- 15. Yamamoto, L.T., Horie, S., Takayama, H., et al. Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. (1999) Gen Pharmacol 33(1): 73-81.
- 16. Boyer, E.W., Babu, K.M., Adkins, J.E. Selfâ€