Clinicopathological Features in Endometrial Carcinoma with Mismatch Repair Deficiency among Chinese Women Younger than 50 Years of Age
Shujie Pang1, Cheng Wang2, Haixia Wu1, Jianchan Song1, Yiquan Shi1
Affiliation
1Department of Pathology, The central hospital of gynecology and obstetrics, TJ, China
2Department of Pathology and Laboratory Medicine, Division of Anatomical Pathology, QEII Health Science Center and Dalhousie University, Halifax, NS, Canada
Corresponding Author
Liu,Y. Department of Pathology, The Central Hospital of Gynecology and Obstetrics,Nankai, Tianjin, China. Tel:+86-022-5828-7667; E-mail: yixinliu66@aliyun.com
Citation
Liu, Y. et al. Clinicopathological Features in Endometrial Carcinoma with Mismatch Repair Deficiency among Chinese Women Younger Than 50 Years of Age (2015) J Gynecol Neonatal Biol 1(1): 3-8.
Copy rights
© 2015 Liu, Y. et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License.
Keywords
Endometrial carcinoma; MMR genes; Lynch syndrome (LS); Morphology features; Immunohistochemistry (IHC)
Abstract
Objective: Mismatch repair (MMR) deficiency can lead to genome instability and closely correlated with carcinogenesis of endometrial carcinoma and mainly caused by MMR gene germline mutation known as Lynch syndrome (LS) or epigenetic mechanisms of MLH1 promoter methylation. The main objective of the present study is to correlate the clinicopathological features in endometrial carcinoma from 92 Chinese women younger than 50 years of age with the MMR status.
Methods: In total 92 cases of consecutive hysterectomy specimens with EC in patients younger than 50 years of age were analyzed. We performed immunohistochemistry staining for 4 MMR proteins and two groups (MMR deficiency group and MMR normal group) were subdivided according to the outcome of immunohistochemistry staining.
Results: 38 cases (42%) with the negative expression of MMR proteins are selected for analysis of their clinical and pathological features. This group included 34 cases of endometrioid adenocarcinoma, one case of clear cell adenocarcinoma, three cases of the mixed types including clear cell, serous and neuroendocrine carcinoma mixed with classic endometrioid adenocarcinoma respectively. In addition to squamous differentiation, 29% cases also exhibit other differentiations including mucinous, clear cell, a lot of papillary structures, dedifferentiated, and secretory types. There are 10 cases showing features of peritumoral and tumor infiltrating lymphocytes. Seven cases have synchronous carcinoma of other sites besides the uterus.
Conclusions: The MMR deficient group showed more morphological differentiations including mucinous differentiation, frequent tumor infiltrating lymphocytes, and more frequent synchronous carcinomas of other sites than tumors with a normal immunophenotype of the MMR genes. Features of dedifferentiated EC originated in the lower uterine segment were not identified in our study. Endometrial carcinoma in Chinese patients younger than 50 years of age is more likely to be LS associated carcinoma.
Introduction
The DNA mismatch repair (MMR) system is known for recognizing and fixing errors during replication and preserving genome stability. Defects of the MMR system can lead to genome instability and is involved in carcinogenesis with a mutator phenotype tumor[1]. It is reported to be associated with 10– 15% of colorectal cancer and up to 30% of endometrial cancers[2]. The MMR deficiency, mainly caused by germline mutations in 4 MMR gene or epigenetic mechanisms of MLH1 promoter methylation, can be manifested as loss of expression of one or more MMR proteins.
Germline mutations in MMR genes can give rise to the autosomal dominant disease[3,4], Lynch Syndrome (LS), previously referred as hereditary nonpolyposis colon cancer (HNPCC). The lifetime cumulative risk of endometrial carcinoma in women with Lynch syndrome is as high as 40% to 60%[5,6], which equals to or exceed the risk of colorectal carcinoma. Endometrial carcinoma in more than half of the cases is usually the first primary tumor[7-10]. Correctly recognizing this disease is significantly important for patient and their family members to decrease the cancer risk. It is suggested that the association of tumor morphology and MMR protein status can enhance the detection of Lynch syndrome[4,11].
The objective of the present study is to correlate the clinicopathological features (including stage, tumor type, grade and morphology features) in 92 consecutive endometrial carcinoma from Chinese women younger than 50 years of age with tumor's the MMR status (including MLH1, PMS2, MSH2 and MSH6).
Materials and Methods
Sample Selection
Resection specimens were obtained from 92 patients who underwent surgical resection of endometrial carcinoma at the Central Hospital of Gynecology and Obstetrics (Tianjin, China) from 2011 to 2014. All patients involved have signed an informed consent, which permitted the use of their specimens for our present study.
Histopathologic Review of Tumors
For all tumors, all hematoxylin-stained slides were reviewed independently by two observers. The following variables were assessed: Tumor grade (low grade and high grade), histological subtype (endometrioid, nonendometrioid, mixed type), morphology features (nonsquamous differentiation, features of peritumoral or tumorin filtrating lymphocytes), the presence or absence of histo- prognostic criteria (such as perineural EP or embols or vessel invasion EV). Tumors were staged according to the tumor-node-metastasis (TNM) staging systems (AJCC 7th Ed.)
Immunohistochemistry Procedure
These samples were fixed in 10% formaldehyde solution, dehydrated, and placed in paraffin for histological analyzes. Four-micrometer-thick sections were prepared and mounted on poly-L-lysine-coated slides. Briefly, slides were deparaffinized, and endogenous peroxidase activity was blocked by incubation with 3% H2O2. Heat-induced antigen retrieval was performed using the Ventana CC1 mild reagent (Ventana Medical Systems) for 30 to 60 min. After treatment with 10% normal goat serum to block nonspecific protein binding, pre-diluted primary antibodies against MLH1, MSH2, MSH6, and PMS2 were applied, followed by incubation with horseradish peroxidase-conjugated multimer antibody reagent. The antigen-antibody reaction was visualized using diaminobenzidine as a chromogen.
Immunohistochemistry (IHC) analysis was performed using the following monoclonal antibodies: anti-MLH1, anti-MSH2, anti-MSH6, and anti-PMS2 (Zhongshan biotechnology limited company, Beijing, China). Normal endometrial tissue and stromal cells served as an internal positive control for staining. Completely Loss of expression was recorded when nuclear staining was absent in malignant cells, but preserved in normal epithelial and stromal cells. Two observers assessed all cases independently.
Statistical Analysis
We used SPSS software (Version 16) for the statistical analysis. The x square test was used to determine the statistic significance concerning the difference of MMR protein expression based on immunostaining in normal tissue and primary tumors. The correlation between the MMR protein expression and clinical and pathologic variables of tumors are also determined in a similar manner.
Results
Summary of MMR gene expression in Chinese patients less than 50 years old with endometrial carcinoma (Table 1) Totally, 92 cases of endometrial carcinoma in patients younger than 50 years were identified within the study period. At least one MMR protein was absent in 38 (42%) of the 92 tumors investigated. The MSH2 loss was the most common abnormality, followed by the absence of MSH6 and PMS2. Combined loss of MLH1 and PMS2 occurred in 7 cases (18.4%) while isolated PMS2 loss occurred in 7 cases. Combined loss of MSH2 and MSH6 occurred in 15 cases (39.4%) while isolated loss of MSH2 or MSH6 occurred in 4 and 2 cases respectively. Additional three cases exhibited combined loss of PMS2, MSH2.
Table 1: Distribution of MMR deficiency in patients younger than 50 years of age
| Age | Loss of MLH1 | Loss of PMS2 | Loss of MSH2 | Loss of MSH6 |
|---|---|---|---|---|
| < = 50 | 7 | 17 | 22 | 17 |
| 40- 50 | 5 | 11 | 12 | 12 |
| < = 40 | 2 | 6 | 10 | 5 |
Patient Characteristics in Endometrial Carcinoma with MMR Deficiency
Overall patient age ranged from 24 to 50 years (median 40) in 38 cases of endometrial carcinoma with MMR deficiency. There were 15 (38%) patients who are younger than 40 years while the percent was slightly lower in the normal MMR groups (33%). The majority of patients presented with stage 1a disease (26 cases, 67%), while 6 (15.7%), 2 (5.3%), and 4 (10.5%) patients presented with stage 1b, stage 2, and stage 3 or 4 disease, respectively. Only five patients with the loss of IHC-MMR showed personal and/or family history (13%). The majority of our patients with the loss of MMR protein expression are absent of personal or family history (33/38) of LS-associated tumors.
Morphology Features
34 cases (92%) of Endometrioid adenocarcinoma were identified in the MMR deficiency group. Four cases of Non-endometrioid carcinoma were identified, including one case of clear cell carcinoma, three cases of mixed types of clear cell, serous and neuroendocrine carcinoma mixed with classic endometrioid adenocarcinoma respectively. The number of type II carcinoma or non-endometrioid carcinoma is statistically higher in the MMR deficient group than in the standard MMR group (four cases versus one case).
The endometrial carcinomas were subtyped into low grade (including FIGO grade 1 or 2 endometrioid adenocarcinoma) and high grade endometrial carcinoma[12] (HGEC, consisting of FIGO grade 3 endometrioid carcinoma, dedifferentiated carcinoma and type II carcinoma). Among the 38 endometrial adenocarcinoma with the MMR deficiency, 29 cases (76.3%) were low grade and nine cases (23.7%) were high grade. While in the normal MMR group, high-grade cases accounting for 28.5% and no statistical significance was found between these two groups.
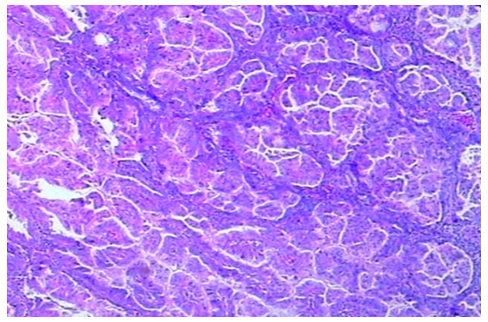
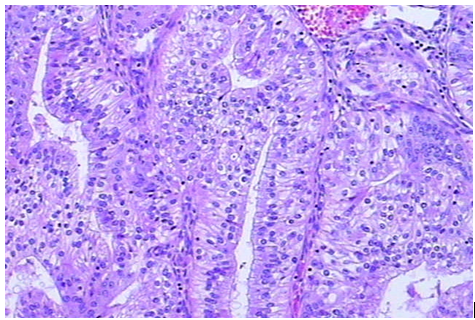
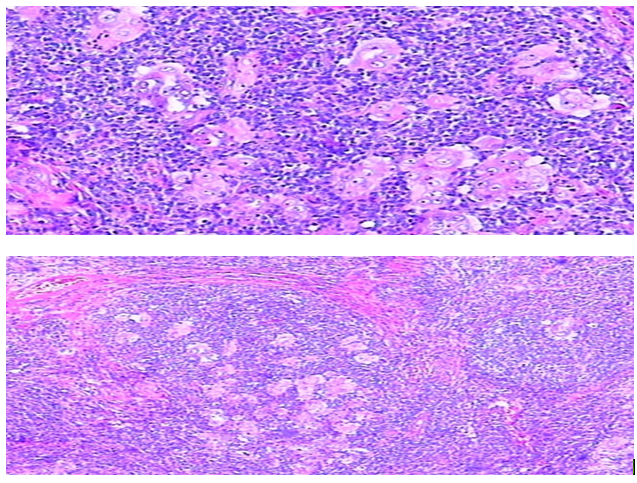
There are 13 cases (34.2%) of endometrial carcinoma with MMR deficiency displaying non-squamous differentiations. There are seven cases showing mucinous differentiation, another three cases showing clear cell, serous or villoglandular differentiation respectively, one case displaying a lot of papillary structures (Figure 1), one case showing secretory adenocarcinoma (Figure 2) and one case of dedifferentiated carcinoma (Figure 3). The number of cases with various non-squamous differentiations in the MMR deficient group is statistically different than that in cases showing normal MMR expression. There are 10 cases exhibiting features of peritumoral or tumor infiltrating lymphocytes (Figure 4).
Other Histological Features
Six cases (16%) of deep myometrial invasion and seven cases (18%) of lymphovascular space invasion were identified. No statistical significance was found between the MMR deficient group and normal MMR counterparts.
Four cases (10.5%) of endometrial carcinoma appeared to be originated from the lower uterine segment and additional 13 cases (34.2%) involved the mucosal or myometrium of the lower uterine segment. Although not statistically significant, the ratio of endometrial carcinoma involving lower uterine segment is higher than that (16.7%) in the normal MMR counterpart.
Seven MMR deficient patients have synchronous tumors in other sites besides the uterus. Four cases are concurrent ovarian endometrioid carcinoma or clear cell carcinoma; two cases are with rectal or colonic cancer. The last case was occurring together with, interestingly, pelvic and abdominal multifocal gastrointestinal stromal tumor (GIST).
Table 2: Relationship between clinicopathological features and MMR status
| characteristics | MMR deficiency Group | Normal MMR group | P value |
|---|---|---|---|
| N | 38 | 54 | |
| Age younger than 40 | 15 | 18 | 0.545 |
| Clinical stage | 0.222 | ||
| Stage Ia | 26 | 43 | |
| Stage Ib and above | 12 | 11 | |
| Deep myometrium invasion | 6 | 6 | 0.512 |
| Lymphovascular invasion | 9 | 6 | 0.108 |
| Histological subtype | 0.071 | ||
| Endometrioid | 34 | 53 | |
| Nonendometrioid | 4 | 1 | |
| Histological grade | 0.893 | ||
| LGEC | 30 | 42 | |
| HGEC | 8 | 12 | |
| Nonsquamous differentiations | 13 | 7 | 0.043* |
| Tumor heterogeneity | 1 | 1 | 0.801 |
| Peritumoral and tumor infiltrating lymphocytes | 10 | 4 | 0.013* |
| Origin from lower uterine segment | 4 | 6 | 0.929 |
| Involvement of lower uterine segment | 13 | 9 | 0.052 |
| Synchronous carcinoma in other sites | 7 | 1 | 0.005* |
Discussion
We have analyzed a cohort of 92 consecutive hysterectomy specimens with endometrial carcinoma in patients younger than 50 years of age and revealed that the rate of abnormal MMR protein expression by IHC is 41.3% (38/92). The rate of loss of expression of MMR protein in unselective endometrial carcinoma is proximately 22.4-35.3% according to some literatures[7,13,14]. In women less than 50 years old, the incidence of MMR loss ranges from 16.2% to 34%[11,15,16] in western countries. However, our rate is slightly higher than that being reported, which may be ascribed to the differences in the ethnic backgrounds of patient populations or case selections[17].
In our study, only 7 cases (18.4%) showed combined loss of MLH1 and PMS2. This rate is significantly lower than that in unselected endometrial carcinoma, which is usually in the range of 58.2%-77.3[7,13]. The difference may be due to the distribution discrepancies of MMR protein expression in patients younger than 50 years of age. Due to the fact that up to 77% of endometrial carcinoma with MMR deficiency[13,18] is associated with epigenetic mechanisms of MLH1 promoter methylation silencing transcription[19,20], the low incidence rate of loss of MLH1 in our cohort signified the low methylation rate in women before 50 years. A report[21] about Microsatellite instability and DNA methylation in endometrial tumors demonstrated that the unmethylated rate in women younger than 50 years with MSI increased to 62%, significantly higher than that of 17% in older women. Our findings are consistent with that younger woman with MMR deficiency exhibited low methylation rate.
Deficient MMR activity would account for the accumulation of mutations throughout the genome[22-25]. In fact, Endometrial carcinoma is diagnosed at an overall significantly earlier age in LS patients[26]. In our study, Loss of both MSH2/MSH6 was detected in 15 patients (39.4%). There are 13 (34.2%) cases showing isolated loss of MSH2, MSH6, or PMS2 protein expression. Three cases, with both losses of MSH2 and PMS2, were found. All these cases accounting for 82.6% were considered suspicious of LS and suitable for genetic testing. Another seven cases with loss of MLH1 expression should be tested first for MLH1 methylation, and unmethylated case were also considered suspicious of LS. Our data further suggested that Chinese women less than 50 have high propensity for LS.
Our study confirms that the majority (93%) of endometrial carcinoma with MMR deficiency is the endometrioid type, however, the number of type II carcinoma or non-endometrioid carcinoma is statistically higher in MMR deficient group than normal MMR group (4 cases versus one case), consistent with the finding of Ryan et al[10] that there was a trend toward LS patients having more nonendometrioid tumors. In addition, mixed type endometrial carcinoma with a unique mix of type I and type II cancer is more frequent in cases with MMR deficiency.
As for relation between MMR deficiency and histo-prognostic variables remains unclear[20,27-29]. Some investigations suggested MMR deficient endometrial carcinoma is associated with tumorgenesis and lymphovascular invasion. However, our data did not provide support for the association between MMR deficiency and high-grade endometrial carcinoma, deep myometrium and lymphovascular invasion.
One apparent morphology feature that is not well reported in the previous studies is that the endometrial carcinoma with MMR deficiency is more likely to display various non-squamous differentiations than the normal MMR group. There are 13 cases (34.2% of all MMR deficiency endometrial carcinoma) consists of various non-squamous differentiations. Of these, seven cases showed mucinous differentiation, one case showed clear cell differentiation, one case with serous differentiation, one case with villoglandular differentiation, one case with a lot of papillary structures, one case of secretory adenocarcinoma and one of dedifferentiated carcinoma. The number of cases with various non-squamous differentiations is statistically more than that in the normal MMR expression group. We observed that the mucinous differentiation is more frequent in the MMR deficient group, and this unusual finding may deserve future investigation.
Several studies[10,30] have reported one of the constant features seen in MMR deficiency associated tumors is the host inflammatory response, which can be separated into "(a) peritumoral lymphocytes, defined as lymphocytic aggregates apparent at a scanning magnification, and (b) the presence of tumor infiltrating lymphocytes, defined as lymphocytes within tumor cell nests or glands (a score of at least 40 lymphocytes per 10 high-power fields indicates a positive finding" [31]. In our study, there are ten cases (26.3%) with MMR deficiency demonstrating the presence of peritumoral or tumor infiltrating lymphocytes. Although the number is lower than the study of Van Den Bos M et al[31], which showed 34% of endometrial carcinoma with LS had features of peritumoral lymphocytes; it is still statistically different than that in the normal MMR group.
An additional feature that deserves more attention is the tumor heterogeneity, which is often defined as dedifferentiated EC, which comprises FIGO grade 1 or 2 endometrioid carcinomas and undifferentiated carcinomas. One earlier study reported the features of tumor heterogeneity, which was not well accepted by some authors because the actual incidence of LS associated carcinoma having dedifferentiated component is only 5%[10,26]. Our data showed only one case (3%) of the MMR deficient endometrial carcinoma with dedifferentiated carcinoma component, consistent with the view that tumor heterogeneity is likely not a particular feature for LS.
The correlation between the anatomic origins of endometrial carcinoma and Lynch syndrome is controversial. Some investigators identified a positive "correlation between the anatomic origin of the cancer and individuals with LS: Up to 29% of patients with ECs centered in the lower uterine segment had LS"[32]. Another group reported the incidence of the lower uterine segment involvement in the LS patients was merely 5.3%[33]. Our study focused on Chinese women with MMR deficiency younger than 50 years of age. We have found about 10% of the endometrial carcinoma in the MMR deficiency group are originated in the lower uterine segment, which showed no statistical difference compared with that in the normal MMR group. However, the actual incidence of involvement of the lower uterine segment was 34%, which is statistically significant different than that in the normal MMR group.
In summary, the MMR deficient group showed more non-squamous histomorphological differentiations including cases with mucinous differentiation. The MMR deficient group is also more likely to have tumor infiltrating lymphocytes and more frequent synchronous carcinomas of other sites than tumors with a standard MMR immunophenotype. Features of dedifferentiated EC and features of frequently originated from the lower uterine segment were not found in our study. The majority of our patients with the loss of MMR protein expression does not have a personal or family history (33/38) of LS-associated tumors.
References
- 1. Masuda, K., Banno, K., Yanokura, M., et al. Relationship between DNA Mismatch Repair Deficiency and Endometrial Cancer. (2011) Molecular biology international.
- 2. Guillotin, D., Martin, S.A. Exploiting DNA mismatch repair deficiency as a therapeutic strategy. ( 2014) Experimental cell research 329(1): 110- 115.
- 3. Mills, A.M., Liou, S., Ford, J.M., et al. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. ( 2014) The American journal of surgical pathology 38(11): 1501- 1509.
- 4. Walsh, C.S., Blum, A., Walts, A., et al. Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. ( 2010) Gynecologic oncology 116(3): 516- 521.
- 5. Pang, S., Guo, D. Lynch syndrom associated endometrial carcinoma. (2012) Zhonghua Bing li xue za zhi 41(7): 494- 497.
- 6. Stoffel, E., Mukherjee, B., Raymond, V.M., et al. Calculation of risk of colorectal and endometrial cancer among patients with Lynch syndrome. (2009) Gastroenterology 137(5): 1621- 1627.
- 7. Egoavil, C., Alenda, C., Castillejo, A., et al. Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. (2013) PloS one 8(11): e79737.
- 8. Ferguson, S.E., Aronson, M., Pollett, A., et al. Performance characteristics of screening strategies for Lynch syndrome in unselected women with newly diagnosed endometrial cancer who have undergone universal germline mutation testing. ( 2014) Cancer 120(24): 3932- 3939.
- 9. Lu, K.H., Dinh, M., Kohlmann, W., et al. Gynecologic cancer as a "sentinel cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. (2005) Obstetrics and gynecology 105(3): 569- 574.
- 10. Ryan, P., Mulligan, A.M., Aronson, M., et al. Comparison of clinical schemas and morphologic features in predicting Lynch syndrome in mutation-positive patients with endometrial cancer encountered in the context of familial gastrointestinal cancer registries. (2012) Cancer 118(3): 681- 688.
- 11. Garg, K., Leitao, M.M., Kauff, N.D., et al. Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. (2009) The American journal of surgical pathology 33(6): 925- 933.
- 12. Tafe, L.J., Garg, K., Chew, I., et al. Endometrial and ovarian carcinomas with undifferentiated components: clinically aggressive and frequently underrecognized neoplasms. ( 2010) Mod pathol 23(6): 781- 789.
- 13. Buchanan, D.D., Tan, Y.Y., Walsh, M.D., et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. (2014) J clin oncol: 32(2): 90- 100.
- 14. Joehlin-Price, A.S., Perrino, C.M., Stephens, J., et al. Mismatch repair protein expression in 1049 endometrial carcinomas, associations with body mass index, and other clinicopathologic variables. (2014) Gynecol oncol 133(1): 43- 47.
- 15. Grzankowski, K.S., Shimizu, D.M., Kimata, C., et al. Clinical and pathologic features of young endometrial cancer patients with loss of mismatch repair expression. (2012) Gynecol oncol 126(3): 408-412.
- 16. Matthews, K.S., Estes, J.M., Conner, M.G., et al. Lynch syndrome in women less than 50 years of age with endometrial cancer. (2008) Obstet Gynecol 111(5): 1161- 1166.
- 17. Wu, H.X., Song, J.C., Shi, Y.Q., et al. Mismatch repair gene expression in women youger than 50 years of age. (2012) Zhonghuabinglixuezazhi 41(11): 733- 6.
- 18. Zighelboim, I., Goodfellow, P.J., Gao, F., et al. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. (2007) Journal of clinical oncology: Official journal of the American Society of Clinical Oncology 25(15): 2042- 8.
- 19. Peltomaki, P. Epigenetic mechanisms in the pathogenesis of Lynch syndrome. (2014) Clinical genetics 85(5): 403- 412.
- 20. Kanopiene, D., Smailyte, G., Vidugiriene, J., et al. Impact of microsatellite instability on survival of endometrial cancer patients. (2014) Medicina 50(4): 216- 221.
- 21. Zauber, N.P., Denehy, T.R., Taylor, R.R., et al. Microsatellite instability and DNA methylation of endometrial tumors and clinical features in young women compared with older women. (2010) Int J Gynecol Cancer 20(9): 1549- 1556.
- 22. Le Gallo, M., Bell, D.W. The emerging genomic landscape of endometrial cancer. (2014) Clin Chem 60(1): 98- 110.
- 23. Cancer Genome Atlas Research Network., Kandoth, C., Schultz, N., et al. Integrated genomic characterization of endometrial carcinoma. (2013) Nature 497(7447): 67- 73.
- 24. Konstantinopoulos, P.A., Matulonis, U.A. POLE mutations as an alternative pathway for microsatellite instability in endometrial cancer: implications for Lynch syndrome testing. (2015) Cancer 121(3): 331- 334.
- 25. Billingsley, C.C., Cohn, D.E., Mutch, D.G., et al. Polymerase varepsilon (POLE) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. (2015) Cancer 121(3): 386- 394.
- 26. Broaddus, R.R., Lynch, H.T., Chen, L.M., et al. Pathologic features of endometrial carcinoma associated with HNPCC: a comparison with sporadic endometrial carcinoma. (2006) Cancer 106(1): 87- 94.
- 27. Kato, M., Takano, M., Miyamoto, M., et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. (2015) J Gynecol Oncol 26(1): 40- 45.
- 28. Nelson, G.S., Pink, A., Lee, S., et al. MMR deficiency is common in high-grade endometrioid carcinomas and is associated with an unfavorable outcome. (2013 ) Gynecologic oncology 131(2): 309- 314.
- 29. Ruiz, I., Martin-Arruti, M., Lopez-Lopez, E., et al. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. (2014) Gynecologic oncology 134(1): 20- 23.
- 30. Rabban, J.T., Calkins, S.M., Karnezis, A.N., et al. Association of tumor morphology with mismatch-repair protein status in older endometrial cancer patients: implications for universal versus selective screening strategies for Lynch syndrome. (2014) The American journal of surgical pathology 38(6): 793- 800.
- 31. van den Bos, M., van den Hoven, M., Jongejan, E., et al. More differences between HNPCC-related and sporadic carcinomas from the endometrium as compared to the colon. (2004) The American journal of surgical pathology 28(6): 706- 11.
- 32. Westin, S.N., Lacour, R.A., Urbauer, D.L., et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. (2008) Journal of clinical oncology: official journal of the American Society of Clinical Oncology 26(36): 5965- 5971.
- 33. Carcangiu, M.L., Radice, P., Casalini, P., et al. Lynch syndrome--related endometrial carcinomas show a high frequency of nonendometrioid types and of high FIGO grade endometrioid types. (2010) International journal of surgical pathology 18(1): 21- 26.