Comparison between Capillary and Venous Blood Sampling for Lipoprotein Lipase Activity Measuring
Eva Pardina1, Marta Cubells2, David Ricart-Jané1, Juan A. Baena-Fustegueras3, Julia Peinado-Onsurbe1*
Affiliation
1 Departament de Bioquímica i Biomedicina Molecular, Facultat de Biologia, Universitat de Barcelona, Spain
2Research Nurse at Hospital Sant Joan de Déu, Barcelona, Spain
3 Unitat de Cirurgia, Hospital Universitari Arnau de Vilanova, Universitat de Lleida, Spain
Corresponding Author
Dr. Julia Peinado-Onsurbe, Departament de Bioquímica i Biomedicina Molecular, Facultat de Biologia, Universitat de Barcelona, Av. Diagonal 643, 08028 Barcelona, Spain, Tel: +34-934021524/48/ Fax: +34-934021559; E-mail: jpeinado@ub.edu
Citation
Peinado-Onsurbe, J., et al. Comparison between Capillary and Venous Blood Sampling for Lipoprotein Lipase Activity Measuring. (2018) Int J Hematol Ther 4(2): 36- 44.
Copy rights
© 2018 Peinado-Onsurbe, J. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
Introduction: Although the relationship between capillary and venous has been described for other parameters (e.g., haematocrit and glucose), the relationship between these two blood sources for lipoprotein lipase has never been described.
Methods: Capillary and venous pre-heparin blood were obtained from 245 people who were healthy and had a normal-weight. The subjects were compared in such circumstances as fasting, stress, and different times of the year, also were compared to plasma samples obtained from obese individuals (BMI > 40 Kg / m²).
Results: The existing correlation (rho = 0.607) between capillary LPL activity (0.56 ± 0.04 mU / mL plasma) and venous LPL activity (0.39 ± 0.03 mU / mL plasma) has a slope of 0.533 and is significant (p < 0.0001). In all situations, it has been observed that whether a subject was a man or woman, fasting or not, with or without stress, the capillary LPL activity is always significantly (p < 0.0001) higher compared to venous blood. Different plasma lipid parameters and indices considered important for determining the risk of cardiovascular diseases (TC / cHDL, cLDL / cHDL,.) correlate better with capillary LPL activity than with venous LPL activity. The differences between capillary LPL activity and venous LPL activity are lost or reversed for obese individuals.
Conclusion: Plasma capillary LPL activity is higher than plasma venous LPL activity, is easily obtainable and is highly sensitive to patho- and physiological changes. Evaluation of the LPL from capillary enables researchers to assess LPL, even under circumstances in which it is not detected in veins.
Introduction
Lipoprotein lipase (LPL, EC 3.1.1.34) is a glycoprotein enzyme with a dimeric active form that is synthesized in the parenchymal cells of the tissues, after which it is translocated through the extracellular matrix and across endothelial cells to the capillary lumen[1].
Many studies indicate that heparin accelerates the flow of LPL to the liver and causes a temporary decrease in the enzyme in peripheral tissues[2]. LPL catalyses the hydrolysis of triacylglycerides (TAG) present in very low density lipoproteins (VLDL) and in chylomicrons (CM). The free fatty acids (FFA) produced by enzymatic catalysis are captured by different tissues and incorporated into cellular metabolism[3]. LPL is involved not only in the metabolism of lipoproteins but also in such conditions as obesity, diabetes, hypertriglyceridemia, atherosclerosis, and liver steatosis[4,5]. LPL is an enzyme that is regulated translational and post-translationally, its activity varies depending on such factors as fasting status, sex, stress, and time of year[6-8]. However, despite the enormous importance of LPL on lipid metabolism, there is no research that compares the activity of this enzyme between vein and capillary blood.
The composition of venous and capillary blood is not the same[9]. There are differences between capillary and venous blood in such parameters as the haematocrit[10], in the content of haemoglobin[11], and in glucose[12]. Venous blood is obtained through direct puncture of a vein, which is most often performed in the antecubital area of the arm or the back (top) of the hand and is the specimen of choice for most routine laboratory tests. Capillary blood is obtained from capillary beds that include the smallest veins (venules) and arteries (arterioles) of the circulatory system. The venules and arterioles join in capillary beds to form a mixture of venous and arterial blood along with interstitial and intracelular fluids. Other researchers believe that depending on certain parameters, and despite differences, the samples could be interchangeable, as with CD4 cell counting[13].
Capillary blood sampling, which refers to sampling blood from a puncture on the finger, heel or an earlobe, is increasingly common in medicine. This approach has several advantages over venous blood sampling: it is less invasive, it requires smaller amounts of blood and it can be performed quickly and easily. Capillary blood is often collected from newborns, young children, diabetic patients, elderly patients with fragile veins, and severely burned patients[14].
In this work, we investigated whether LPL activity is the same in two types of blood samples (capillary and venous) extracted from young and healthy individuals. We studied whether there are differences between sexes, as well as the effects of fasting or stress over time, and we compared the results to the outcomes for obese individuals.
Methods
In a cross-sectional study, we collected blood from 245 healthy people (students, 110 men and 135 women), aged 21.6 ± 0.3 years and with a BMI ≤ 25 kg/m2 who were euthyroid, had a normal-weight, were normotensive, and non-diabetic through annual blood bank donor campaigns at different faculties of the University of Barcelona. All subjects gave their written informed consent to participate, and the study protocol was accepted by the blood bank ethics committee. Before obtaining the blood, the students responded to a small survey regarding such questions as whether they took medication, whether they had had food within a certain number of hours and whether they were stressed.
A group of 34 morbid obese patients (24 women and 10 men) between 27 and 61-year-old with a BMI > 40 kg/m2 were recruited from the Valld’Hebron Hospital in Barcelona, Spain according to guidelines of the Spanish consensus for the diagnosis of obesity[15]. The study protocol was accepted by the hospital ethics committee, and all subjects gave their written informed consent to participate. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
First, we obtained capillary blood through puncture of the index finger (preferably of the non-dominant hand) with a disposable lancet. The skin was cleaned and disinfected with alcohol prior to the puncture. Capillary blood was collected in Eppendorf tubes (Microvette® CB 300 K2E, Sarstedt) with spray-coated K2EDTA as anticoagulant and a small hole in its lower end such that the blood penetrates the tube through capillary action. Care was taken to avoid any pressure on the finger, which can result in haemodilution due to the inclusion of interstitial and intracellular fluids. We attempted to collect an approximate volume of 50 to 60 μL.
A venous blood sample was obtained by puncturing the middle vein of the antecubital region after the area was disinfected. Blood is collected in vacutainer tubes (BD-Vacutainer model K2E, Fisher Scientific) with spray-coated K2EDTA as anticoagulant and with a capacity of 5 mL. The vacutainer tube was inverted to ensure adequate mixture with the anticoagulant.
All samples from non-fasting students and fasted obese individuals were taken between 8:00 and 11:00 a.m. in four different months (March, May, October and December) of the year. The plasma was separated immediately by centrifugation, and aliquots were frozen at -80°C for subsequent analysis.
Methods involving the use of TAG containing radiolabelled acyl chains are highly specific and sensitive lipase assays[16,17]. In our experimental procedure, LPL was assayed as previously described by Ballart et al[18]. The assay mixture contained 0.6 mM glycerol tri[9,10(n)-3H]oleate (12 Ci/mol), 50 mM MgCl2, 0.05% FFA-BSA, 3% (vol/vol) preheated serum as an apo C-II source (preheated at 52°C for 1 h to inactivate lipases), 25 mM PIPES (piperazine-N,N′-bis 2-ethanesulfonic acid), at pH 7.5, and 0.007 mL of the preheparin plasma sample in a final volume of 0.067 mL. After incubation for 30 min at 25°C, the reaction was stopped, FFA was extracted, and [3H]oleate was quantified. Production of 1 μmol oleate per min is defined as 1 U LPL. When LPL was assayed in the plasma or capillary (which contains hepatic lipase (HL) in addition to LPL), HL activity was inhibited before the LPL assay by incubating the sample for 90 min on ice with anti-HL antibodies (1:4), as described previously[4]. The endothelial lipase (EL) has no TAG hydrolase activity when 3% – 5% serum is present in the assay[19]. For all subjects, heparin was not used for the extraction of blood or as an anticoagulant due to the effect this compound has on LPL[20].
Protein and glucose determination
content was determined using a colorimetric assay. Glucose was determined following the instructions of the commercial kit for Glucose (Roche Diagnostics). The mass of the enzyme LPL was measured (triplicated) by a commercial ELISA kit (Sekisui Diagnostics).
Plasma free and total cholesterol (FC and TC, respectively), triacylglycerides (TAG), phospholipids (PL) and free fatty acids (FFA) were enzymatically analysed by commercial kits and processed in accordance with the manufacturer’s recommendations (Wako Laboratory Chemicals).
Results are given as the mean ± SEM (in certain cases when the error is not seen in the graph, it is included in the mean). Normality of the data was tested using the D’Agostino and Pearson omnibus normality test. A Bland and Altman plot was used to plot the mean difference between capillary and venous LPL activity against the mean LPL activity level. This plot provides a graphical presentation of the level of agreement between two methods of assessment by plotting the difference between the two measurements versus the mean for each subject. As the procedure removes most of the variation between subjects and leaves the measurement error, it is expected that the differences will be normally distributed. When there is a high level of agreement, the mean difference will be close to zero, and the confidence intervals for the difference will be narrow[21].
Differences between mean values from capillary and venous blood in students depend on gender, month of the year, fasting, or stress, which were analysed with the non-parametric method of the Kruskal-Wallis test. Individual comparisons were made using Dunn’s Multiple Comparison.
Differences between mean values from capillary and venous blood in students and obese individuals were analysed with a non-parametric Kruskal-Wallis test. Individual comparisons were made using Dunn’s Multiple Comparison.
Correlations between values from capillary and venous blood were determined with the Spearman’s correlation coefficient (rho). Statistical comparisons were significant when p < 0.05. In the correlation, we see that the number of value pairs is only 225 instead of 245 because in certain cases, the vein or capillary samples of some of the students could not be obtained.
All statistical analyses were computed using the GraphPad Prism programme version 4.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com).
Results
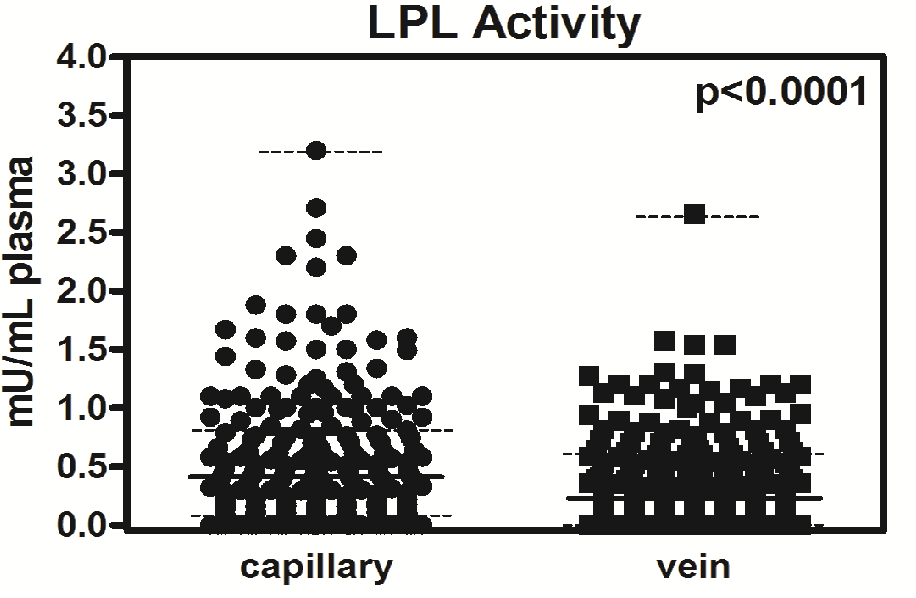
Figure 1 shows the capillary pre-heparin LPL activity (0.56 ± 0.04 mU / mL plasma) and vein activity (0.39 ± 0.03 mU / mL plasma) for the students, the median (0.43 and 0.30 mU / mL, respectively), the minimum value (it is zero, in both cases), the maximum value (3.2 and 2.7 mU / mL, respectively), the 25th percentile (0.09 and 0.03 mU / mL, respectively) and 75% (0.84 and 0.60 mU / mL, respectively). The value of the capillary LPL activity is always greater and significant (p < 0.0001) compared to activity in vein samples.
Figure 1: Median and interquartile range for LPL activity in capillary and vein samples. Activity (n = 245) is expressed as a median (continuous line) and interquartile range (dotted lines). Statistical significance was calculated using a paired t-test (upper right corner).
The intraassay imprecision (CV), determined by replicate analysis of four single sample, was 3.3% for LPL activity. Interassay CVs, determined from 12 separate LPL assays performed over a 6-month period, were 16.9%.
In the Supplementary File Figure 1’ we can see in the histogram that the relative frequency is expressed as a percentage for the LPL activity. The highest relative frequency correlates with the lowest LPL activities. Although we observed maximum values for capillary LPL activity of up to 3.2 mU / mL plasma and in up to 2.6 mU / mL plasma in vein samples, the frequency of these values is very low.
Supplementary File Figure 1’ Histogram of bars for LPL values of the entire population. At the Y axis, we have the relative frequency obtained from dividing the data that have a certain value (LPL activity in plasma, in the X axis) by the total number of data. The clear bars correspond to the capillary plasma and the grey onescorrespondto the venous plasma.
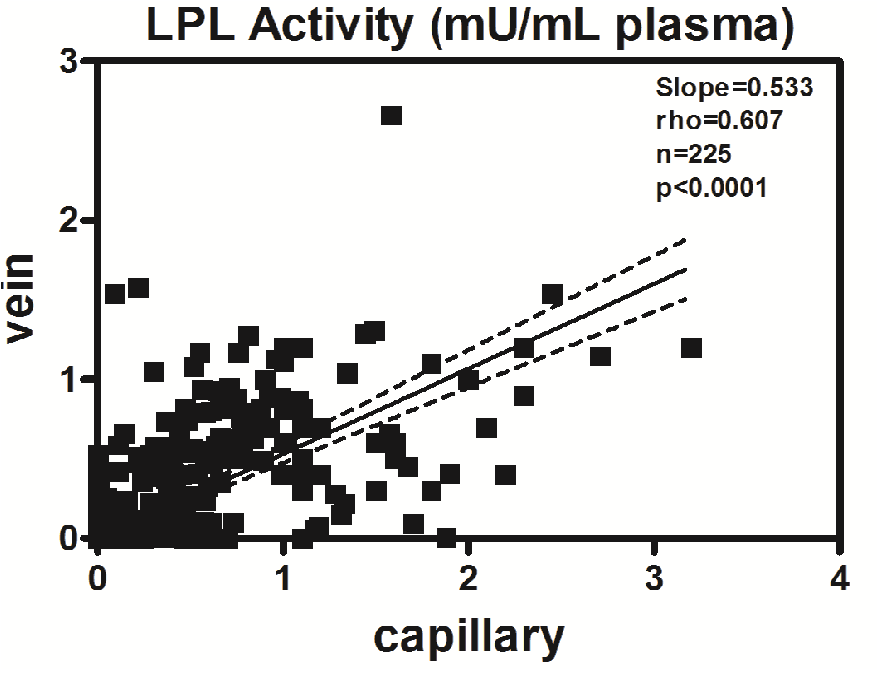
The correlation (rho = 0.607) between the capillary LPL and the vein LPL (Figure 2’) has a slope of 0.533 and is significant (p < 0.0001).
Figure 2: Correlation between LPL activity in capillary and vein samples. Correlation between plasma LPL activity from capillary and vein samples. Spearman rho = 0.607, p < 0.0001; dotted lines indicate 95% confidence intervals.
If the capillary LPL activity is divided into intervals (Supplementary File Figure 2’) and compared with vein values, it is observed that except between the ranges of 0.0 (from 0.0 to 0.199) to 0.2 and 0.2 to 0.4 mU / mL in which the activity value is slightly higher in vein than in the capillary, the values is always higher for the capillary samples than the vein samples. Furthermore, the capillary LPL activity correlates perfectly with the established ranges, whereas is does not correlate for vein activity (rho = 0.7, p < 0.05).
Supplementary File Figure 2’. LPL activity of the entire population in intervals. Results are given as the mean ± SEM. Linear regression between the mean LPL activity and intervals of LPL activity in ranges. Statistical differences between the mean values for capillary (clear bar) or vein (grey bar) samples were assessed using the non-parametric method of the Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. The symbol (°) denotes a difference between capillary and vein samples. Three symbols: P < 0.001.
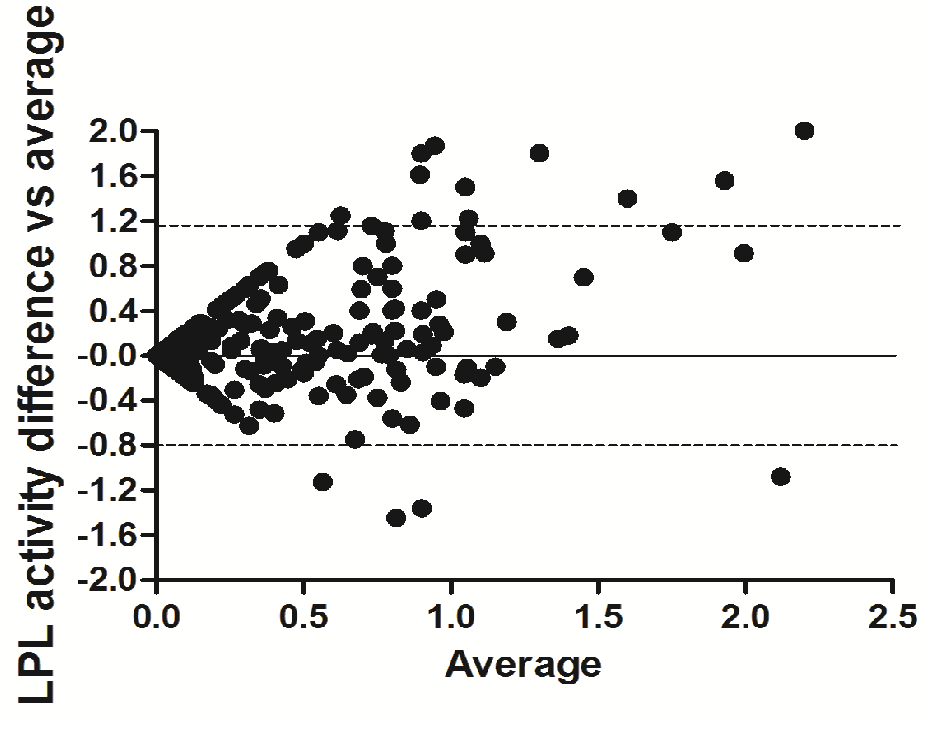
The Bland-Altman difference plot (Figure 3) showed a small (0.16) bias (difference between the means = 16%) with an SD = 0.49, and the 95% limits of agreement was from -0.83 to 1.20. Overall, 94% of the values fell within these limits.
Figure 3: Bland-Altman scatter plot of the differences between capillary and venous blood LPL activity. Solid line represents the mean difference; dashed lines represent 95% limits of agreement (from -0.828 to 1.198). Bias = 0.185; SD of Bias = 0.517.
Arterio-venous differences of the LPL activity according to genre
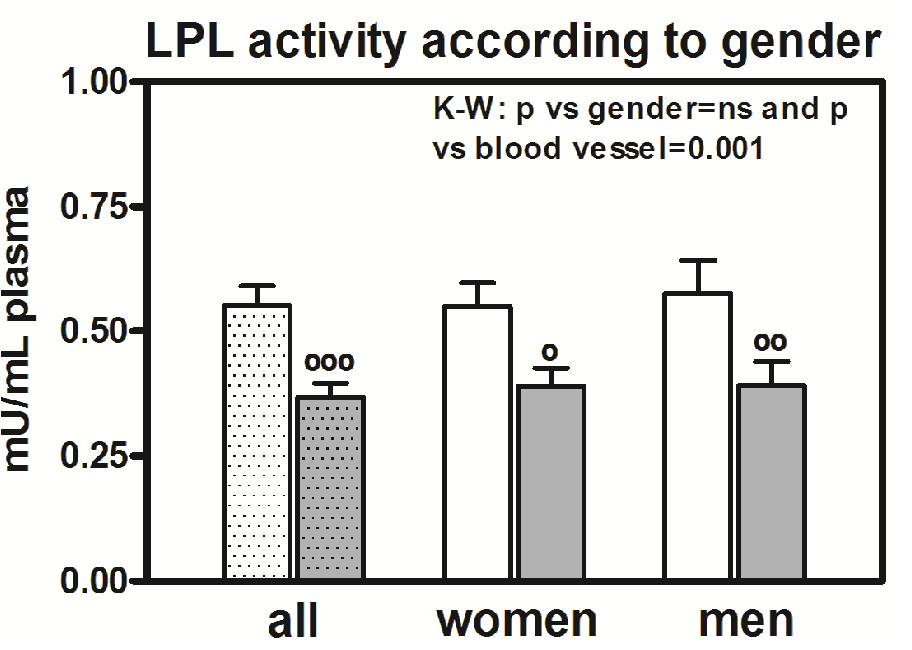
We observed significant differences (p < 0.0001) in LPL activity in capillaries and veins if we consider all the individuals together (Figure 4), and we also observed significant differences if we consider the individuals according to gender (Figure 4). There was a higher difference between the capillary and vein samples in men (p < 0.01) than in women (p < 0.05). However, there are no differences in LPL activity between men and women. The correlation between the capillary and vein samples for women is as significant (rho = 0.5623, p < 0.0001) as it was for men (rho = 0.6075, p < 0.0001).
Figure 4: LPL activity according to gender. Results are given as the mean ± SEM. Statistical differences between mean values for capillary (clear bar) or vein (grey bar) samples for women and capillary or vein samples for men were assessed using the non-parametric method of the Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. The K-W test result is in the right corner of the panel. The dotted bar corresponds to men and women together. Symbol (°) denotes a difference between capillary and vein samples. One symbol: P < 0.05; two symbols: P < 0.01, and three symbols: P < 0.001.
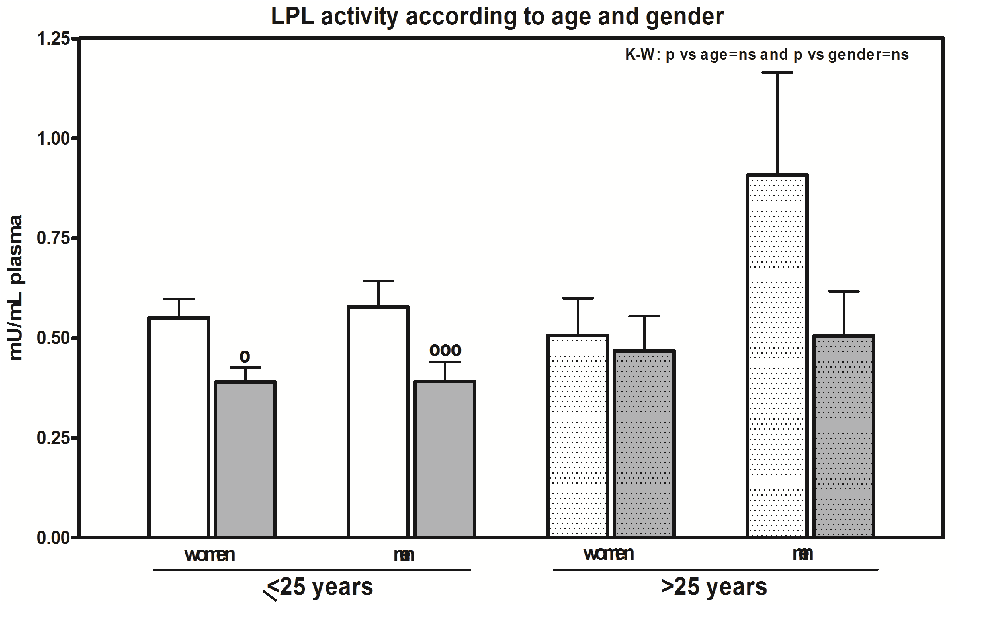
Some of the group (20 people: 10 women and 10 men) at the time of extraction was between 26 and 57 years-old (33.7 ± 7.5 years old), either because they were older students or because they were teachers. Although we observed arterio-venous differences in both women (p < 0.05) and men (p < 0.001) in the group that is less than 25 years old (Figure 5), the differences disappear in the group with ages over 25. In this group, the capillary LPL activity in women ranges from 0 and 0.78 mU / mL plasma, and for men, it was between 0.02 and 2.1 mU / mL plasma, while the activities in veins are similar in the two sexes (0 and 0.93 and 0 and 1 mU / mL plasma, respectively). There are no significant differences between the two age groups or between men and women.
Figure 5: LPL activity according to age and gender. Results are given as the mean ± SEM. Statistical differences between the mean values for capillary (clear bar) or vein (grey bar) samples for women and capillary or vein samples for men who were less ≤ 25 years old or who were assessed using the non-parametric method of the Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. The K-W test result is in the right corner of the panel. The dotted bar corresponds to men and women with an age > 25 years old. The symbol (°) denotes differences between capillary and vein samples. One symbol: P < 0.05; and three symbols: P < 0.001.
The mass of the enzyme measured by a ELISA kit resulted in no differences between capillary and vein neither in men (38.5 ± 3.5 ng / mL plasma) nor in women (45.5 ± 4.3 ng / mL plasma) (data not shown).
Arterio-venous differences of the LPL activity according to the month of extraction
As we mentioned in the methods section, two sample collections were conducted in the Engineer’s School, and two sample collections were conducted in the Faculty of Biology. When we performed the assessment of LPL activity in these groups, we observed there were significant differences (p = 0.0002) between these groups in addition to the corresponding differences between capillary and vein samples. Because there was no apparent reason for there to be differences between these groups, we decided to study at the time of the year each sample collection was conducted. Thus, the month of the year in which the collection was conducted showed that there are no differences in the LPL activity (Supplementary File Figure 3’) between capillary and vein samples (for the months of March and December) or that the activity was higher in May or lower in October compared March or May. Conversely, the people who provided blood in May had a greater LPL activity in both their capillary and vein samples compared to individuals who provided samples in December (6.9 and 3.4 times, respectively).
Supplementary File Figure 3. LPL activity according to month of the year. Results are given as the mean ± SEM. Statistical differences between the mean values for capillary (clear bar) or vein (grey bar) samples and month of the year (March, n = 80; May, n =28; October, n = 86; December, n = 51) for blood extraction were assessed using the non-parametric method of the Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. In the right corner of panel, there is the K-W test result. The symbol (°) denotes differences between capillary and vein samples. The symbol (*) denotes differences between capillary and vein samples in different months. One symbol: P < 0.05; two symbols: P < 0.01; and three symbols: P < 0.001.
This same LPL activity pattern occurred for capillary and vein samples throughout the year, regardless of whether the subjects were men or women (data not shown).
Arterio-venous differences in the LPL activity according to hours of fasting
The tendency for LPL activity in the capillary samples to be higher than in vein samples was preserved, regardless of whether subjects had just eaten or if they had been fasting for several hours (Supplementary File Figure 4’). The ANOVA indicates that there were significant differences both from the effect of hours of fasting (p = 0.0003) and through capillary or venous blood (p = 0.0107). Since the group that did not answer the survey (n = 107) on fasting hours was the most numerous, the differences between capillary and vein samples were more significant (p < 0.001) compared to the other groups.
Supplementary File Figure 4’. LPL activity according to hours of fasting. Results are given as the mean ± SEM. Statistical differences between the mean values for capillary (clear bar) or vein (grey bar) samples and hours of fasting until blood extraction were assessed using the non-parametric method of the Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. The K-W test result is in the right corner of the panel. The dotted bar correspond to people who had not answered the survey about hours of fasting. The symbol (°) denotes differences between capillary and vein samples. The symbol (*) denotes differences between capillary and vein samples depending on hours of fasting. One symbol: P < 0.05; two symbols: P < 0.01; and three symbols: P < 0.001.
Arterio-venous differences of the LPL activity according to stress
It should be noted that arterio-venous differences in LPL activity are still observed whether the survey determined if subjects were stressed or not (Supplementary File Figure 5’), although for two cases, there is only a tendency, and it is only significant in the larger group (n = 126) that did not respond to the survey.
Supplementary File Figure 5’. LPL activity according to stress. Results are given as the mean ± SEM. Statistical differences between the mean values for capillary (clear bar) or vein (grey bar) samples and people who answered yes or no to whether they were stressed at the moment of blood extraction were assessed using the non-parametric Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. In the right corner of panel, there is the K-W test result. The dotted bar corresponds to people who did not answer the stress survey. The symbol (°) denotes differences between capillary and vein samples. The symbol (*) denotes differences between the capillary and vein samples depending on hours of fasting. One symbol: P < 0.05; two symbols: P < 0.01; and three symbols: P < 0.001.
Correlation of capillary and vein LPL activity with different lipid parameters
Supplementary File Table 1’ shows the correlation between different plasma lipid parameters and indices that are considered important for determining the risk of cardiovascular diseases (CT / cHDL, cLDL / cHDL, CT-cHDL, TAG / cHDL). The capillary LPL activity correlates better with more parameters than the LPL activity of veins.
Supplementary File Table 1: Correlation between LPL activity in capillary and vein samples and different parameters.Correlations between plasma LPL activity from capillary and venous samples (n = 225) were determined with the Spearman’s correlation coefficient (rho). Statistical comparisons were significant when p < 0.05. Abbreviations: Gluc, glucose; TAG, triglycerides; PL, phospholipids; FFA, free fatty acid; TC, total cholesterol; FC, free cholesterol; CE, esterified cholesterol; cHDL, HDL cholesterol; cLDL, LDL cholesterol. Ratios or indices between anterior parameters: TC/cHDL; cLDL/cHDL; TC-cHDL; TAG/cHDL. LPL and HL lipoprotein lipase and hepatic lipase. Values in bold are significant. Values in italics are not significant.
| capillary | vein | |||
|---|---|---|---|---|
| rho | p | rho | p | |
| AGE | - 0.029 | 0.637 | 0.127 | 0.051 |
| GLUC | - 0.214 | 0.001 | - 0.088 | 0.186 |
| TAG | 0.178 | 0.008 | 0.258 | 0.000 |
| PL | 0.075 | 0.266 | 0.158 | 0.017 |
| FFA | 0.200 | 0.003 | 0.174 | 0.009 |
| TC | - 0.088 | 0.189 | - 0.052 | 0.432 |
| FC | 0.137 | 0.040 | 0.297 | 0.000 |
| CE | - 0.163 | 0.015 | - 0.203 | 0.002 |
| cHDL | 0.155 | 0.020 | - 0.035 | 0.597 |
| cLDL | - 0.156 | 0.019 | - 0.049 | 0.463 |
| TC/cHDL | - 0.162 | 0.016 | - 0.004 | 0.957 |
| cLDL/cHDL | - 0.169 | 0.012 | 0.005 | 0.946 |
| TC-cHDL | - 0.132 | 0.049 | - 0.047 | 0.481 |
| TAG/cHDL | 0.100 | 0.136 | 0.246 | 0.000 |
| Capillary LPL | 0.612 | 0.000 | ||
| Vein LPL | ||||
| Capillary HL | 0.260 | 0.000 | 0.043 | 0.534 |
| Vein HL | 0.033 | 0.617 | - 0.063 | 0.343 |
Arterio-venous differences of the LPL activity according to gender or month of the year in obese patients.
When we study the arterio-venous differences of LPL activity in morbidly obese patients (Supplementary File Figure 6’), we observe that the LPL activity are lightly higher in vein samples (0.49 ± 0.09 mU / mL plasma) compared to capillary samples (0.41 ± 0.04 mU / mL plasma), but not significant. The ratio between capillary and vein samples in the lean is 2.06 ± 029, whereas for obese individuals, it is 1.04 ± 0.21, which is half the value of the lean samples. There is no difference between the vein samples of lean and obese individuals, but the LPL activity in the capillary samples of lean and obese individuals was slightly different, but not significant (0.56 ± 0.04 vs 0.41 ± 0.04 mU / mL plasma, respectively). The correlation of capillary and vein activity in morbidly obese individuals (r = 0.5998) has a slope = 0.8495 and is significant (p = 0.0012). In the correlation, we see that the number of value pairs is only 26 instead of 34, because in certain cases, the vein or capillary value of some of the obese individuals could not be obtained. The difference in LPL activity in post-heparin plasma between capillary (0.78 ± 0.14, mU / mL plasma) and vein (0.63 ± 0.12, mU / mL plasma, p = 0.0149) remains similar to that observed in thin people. However, we did not have occasion to verify it in the thin individuals.
Supplementary File Figure 6’. LPL activity according to gender in obese people: Results are given as the mean ± SEM. Statistical differences between mean values for capillary (clear bar) or vein (grey bar) samples in women (n = 24) and capillary or vein samples in men (n = 10) with a BMI >40 kg/m² were assessed using the non-parametric Kruskal-Wallis (K-W) test. Individual comparisons were made using Dunn’s Multiple comparisons test. The K-W test result is in the right corner of the panel. The dotted bar corresponds to data for men and women combined. Ns, not significant.
We have not observed significant differences between capillary and vein activity in women or men, but neither was observed between the two sexes. We have observed that there is a correlation between the two types of blood in women (rho = 720, p = 0.001) but not in men. With regard to the time of year in which the extraction was made, the differences among the students observed in summer and autumn did not appear in the obese individuals.
Discussion
This paper shows arterio-venous differences in LPL activity for the first time.
We observed that LPL activity in capillary plasma is 30.4% higher than venous plasma. Overall, 58.2% of the population have LPL activity values in the capillary that oscillate between 0 and 0.4, whereas they are 78.7% for the veins.
In the literature, although it must be said that it is very scarce for LPL activity in pre-heparinic plasma, we obtain values for LPL of 0.5 ± 0.27 mU / mL in the veins of healthy subjects with an average age of 38 years[22], and the error is sufficiently great that a previous measurement must be stated. In other studies, LPL activity, which was also determined in pre-heparinic venous plasma for a population of individuals with an average age of 30 years, was 0.23 ± 0.02 mU / mL of plasma[23]. In this study, we obtained highly similar results, and the method of measuring LPL activity is highly similar.
However, there are other studies that show higher values in men who are healthy and normotriglyceridemic with ages between 39 and 48 years old, and those studies obtained an LPL activity of 1.4 ± 0.9 mU / mL of plasma[24] or 1.54 ± 0.15 mU / mL of plasma[25]. It should be noted that these LPL values in preheparinic plasma have been measured using a method to measure LPL activity with a radioactive substrate based on Intralipid (which is lipid emulsion used for parenteral and clinical feeding), and this approach usually yields notably high activity for both pre- and post-heparinic plasma[26].
Regarding the activity of LPL in human pre-heparinic capillary plasma, we have not identified results in the existing literature with which to compare our data.
Although an attempt was made to avoid massaging the finger to obtain the capillary blood as much as possible, if massage was used, it is likely that the blood sample was more diluted due to the possible release of interstitial and / or intracellular fluids; therefore, the activity would be lower but never higher. One possibility that has not been ruled out and has not been demonstrated in this study is that since LPL is an enzyme that is anchored in the endothelium of the capillaries, more LPL could have been released if massage occurred, although it is assumed that in the fluids would have provided the same proportion of LPL.
In our research group, we observed in rats that depending on the area of blood extraction (e.g., caudal vein, cava vein, aorta artery, or hepatic vein), there are differences in pre-heparin LPL activity value[27].
Other authors have observed in men the skeletal muscle LPL activity is higher in venous plasma than in arterial plasma, and in contrast, plasma vein LPL activity is lower than in arterial plasma in subcutaneous fat tissue[26].
Similarly, there are other authors who also did not observe differences between men and women, as was the case for Watson et al.[22]. Other researchers[28], conversely, did observe differences, although in those cases, the plasma was post-heparinic.
Recently, we have shown for the first time that under physiological conditions, plasma LPL and HL activities, as well as lipids, follow circannual rhythms[8,9]. Since LPL activity in adipose tissue and skeletal muscle is lower in the summer than in winter[29], it could be that more LPL is being released from the tissue in the summer to be eliminated or recycled, and for that reason, we observed higher plasma activity.
The differences in LPL activity between the different months of the year could also be due to physiological changes or changes in the environment. Changes in the environment include the type of diet, physical activity, weather, environmental temperature and periods of light. Physiological changes include changes in body composition (e.g., water balance and thermodynamic variations due to temperature change) or in hormone levels. A study on the effects of seasonal change in lipid levels in the blood found significant differences in the diet between summer and winter[30]. In animal models, high levels of melatonin during the short photoperiod in winter[29] are related to low levels of LPL plasma activity[31,32]. Therefore, in the cold months, in which the photoperiod is shorter, plasma LPL activity is lower, as seen in our data.
We know that there are other factors that can cause plasma LPL activity to increase, such as stress. In our laboratory, in different animal models, we have observed that stress and / or adrenaline increase LPL activity in plasma[7,8,33]. This increase is notably fast, since five minutes have already been seen in plasma[27]. We have also observed an increase in plasma LPL in other types of stress, such as fasting[34], exercise[35], and surgery[36]. It has been shown that there is a variation in the LPL activity levels in humans during the day, whereas the peaks of enhanced LPL activity coincide with post-prandial states, and even higher activities are found in the morning compared to the afternoon[37].
In the case of women with morbid obesity, the tendency for LPL activity to be higher in capillary samples than in vein samples (that we had observed in lean people) is reversed, which is something that is not observed in men with morbid obesity. The variability is lesser in both capillary (women = 62% and men = 45%) and vein (women = 97% and men = 69%) samples in obese individuals compared to lean individuals (capillary: women = 95% and men = 111% and, vein: women = 97% and men = 119%), despite the number of obese individuals is lower.
As we mentioned in the introduction, the composition of venous blood and capillary blood is not the same[9], and the haematocrit is higher in capillary blood compared to venous blood[11], which is also observed for haemoglobin[11], glucose[12] or red blood cells[38]. Several authors consider that the difference found between fingertip-capillary and antecubital-venous samples for estimates of insulin should not be employed interchangeably[39]. Other researchers consider that under certain parameters, and despite the differences that exist, the samples could be interchangeable, as is the case for the CD4 cell count[13]. Although good correlation is the norm between venous- and capillary-derived samples, caution must be exercised in regard to accepting the results as equivalent or using either interchangeably[40].
As we have observed, capillary LPL correlates very significantly with most lipid parameters (e.g., TAG, FFA, FC, cHDL, and cLDL). Given that LPL activity would cause a reduction of TAG plasma and an increase in cHDL that results in protection against the development of atherosclerosis[41], it is even more important to assess the activity with a simple method.
Conclusion
Capillary blood sampling via a finger or earlobe prick may provide advantages over more invasive methods of sampling due to the ease of application, minimal skill requirements, reduced blood volume that is required, and cost-effectiveness[42]. Due to the advantages of extracting blood in a simple way from the capillary, we believe that for LPL, it would be best if samples were always routinely collected through a single system with periodic controls to test LPL vein activity. It would be interesting to perform a similar study of other plasma lipases, such as p.e. hepatic lipase (which is similar to LPL), to determine if their behaviour is similar to that of LPL.
Conflict of interest: The authors have declared that no conflicts of interest exist. The authors who have taken part in this study do not have a relationship with the manufacturers of the drugs involved either in the past or present and did not receive funding from the manufacturers to carry out their research.
Financial support: This work was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (ISCIII) (PI11/01159 and PI15/00190 to JP-O PI15/00332 to JAB-F) and the FEDER Funds of the EU (FondoEuropeo de Desarrollo Regional: “Una manera de hacer Europa”).
Acknowledgements: We would especially like to thank the students and teachers from University of Barcelona who participated in the study. Special thanks also are due to the Blood Bank of the Hospital Clínic de Barcelona (Spain) and to its Haemotherapy and Haemostasis Service, which facilitated blood collection and helped us to collect the samples.
English grammar and language were corrected by American Journal Experts (www.journalexperts.com).
Ethics committee approval: I certify that a statement confirming that appropriate institutional and/or ethics committee approval has been obtained and is stated in the Methods section of the manuscript.
References
- 1. Braun, J.E., Severson, D.L. Regulation of the synthesis, processing and translocation of lipoprotein lipase. (1992) Biochem J 287(Pt 2): 337-347.
- 2. Neuger, L., Vilaro, S., Lopez-Iglesias, C., et al. Effects of heparin on the uptake of lipoprotein lipase in rat liver. (2004) BMC Physiol 4(1): 13.
- 3. Merkel, M., Eckel, R.H., Goldberg, I.J. Lipoprotein lipase: genetics, lipid uptake, and regulation. (2002) J Lipid Res 43(12): 1997-2006.
- 4. Pardina, E., Lecube, A., Llamas, R., et al. Lipoprotein Lipase but Not Hormone-Sensitive Lipase Activities Achieve Normality After Surgically Induced Weight Loss in Morbidly Obese Patients. (2009a) Obes Surg 19(8): 1150-1158.
- 5. Pardina, E., Baena-Fustegueras, J.A., Llamas, R., et al. Lipoprotein lipase expression in livers of morbidly obese patients could be responsible for liver steatosis. (2009b) Obes Surg 19(5): 608-616.
- 6. Galan, X., Llobera, M., Ramírez, I. Lipoprotein lipase and hepatic lipase in Wistar and Sprague-Dawley rat tissues. Differences in the effects of gender and fasting. (1994) Lipids 29(5): 333-336.
- 7. Ricart-Jané, D., Cejudo-Martín, P., Peinado-Onsurbe, J., et al. Changes in lipoprotein lipase modulate tissue energy supply during stress. (2005) J Appl Physiol (1985) 99(4): 1343-1351.
- 8. Cambras, T., Baena-Fustegueras, J.A., Pardina, E., et al. Seasonal variation in plasma lipids and lipases in young healthy humans. (2017) Chronobiol Int 34(9): 1248-1258.
- 9. Neufeld, L., García-Guerra, A.M. Hemoglobin measured by hemocue and a reference method in venous and capillary blood: a validation study. (2002) Salud Publica Mex 44(3): 219-227.
PubMed||CrossRef||Others
- 10. Ramamurthy, R.S., Brans, Y.W. Neonatal polycythemia: I. Criteria for diagnosis and treatment. (1981) Pediatrics 68(2): 168-174.
- 11. Valenzuela, P. Poliglobulia neonatal. Ed. Servicio neonatol. Hospital Clín. Universidad Chile. (2001) 172-177.
PubMed||CrossRef||Others
- 12. McIlroy, D., Banham, N. Comparison of venous glucose to finger-prick glucose in patients with diabetes under hyperbaric hyperoxic conditions: a pilot study. (2013) Diving Hyperb Med 43(4): 226-228.
PubMed||CrossRef||Others
- 13. MacLennan, C.A., van Oosterhout, J.J., White, S.A., et al. Finger-prick blood samples can be used interchangeably with venous samples for CD4 cell counting indicating their potential for use in CD4 rapid tests. (2007) AIDS 21(12): 1643-1645.
- 14. Lenicek Krleza, J., Dorotic, A., Grzunov, A., et al. Capillary blood sampling: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. (2015) Biochem Med (Zagreb) 25(3): 335-358.
- 15. Sociedad Española para el estudio de la Obesidad (SEEDO). SEEDO’2000 consensus for the evaluation of overweight and obesity and the establishment of criteria for therapeutic intervention. (2000) Med Clin 115(15): 587-97.
- 16. Belfrage, P., Fredrikson, G., Olsson, H., et al. Molecular mechanisms for hormonal control of adipose tissue lipolysis. (1985) Int J Obes 9(Suppl 1): 129-135.
PubMed||CrossRef||Others
- 17. Briquet-Laugier, V., Ben-Zeev, O., Doolittle, M.H. Determining lipoprotein lipase and hepatic lipase activity using radiolabeled substrates. (1999) Methods Mol Biol 109: 81-94.
- 18. Ballart, X., Peinado-Onsurbe, J., López-Tejero, D., et al. Isoproterenol increases active lipoprotein lipase in adipocyte medium in rat plasma. (2003) Biochimie 85(10): 971-982.
- 19. McCoy, M.G., Sun, G.S., Marchadier, D. Characterization of the lipolytic activity of endothelial lipase. (2002) J Lipid Res 43(6): 921-929.
PubMed||CrossRef||Others
- 20. Peinado-Onsurbe, J., Soler, C., Galán, X., et al. Involvement of Catecholamines in the effect of fasting on Hepatic Lipase activity in the rat. (1992) Endocrinology 129(5): 2599-2606.
- 21. Bland, J.M., Altman, D.G. Agreed Statistics: Measurement Method Comparison. (2012) Anesthesiology 116(1): 182-185.
- 22. Watson, T.D., Tan, C.E., McConnell, M., et al. Measurement and physiological significance of lipoprotein and hepatic lipase activities in preheparin plasma. (1995) Clin Chem 41(3): 405-412.
- 23. Glaser, D.S., Yost, T.J., Eckel, R.H. Preheparin lipoprotein lipolytic activities: relationship to plasma lipoproteins and postheparin lipolytic activities. (1992) J Lipid Res 33(2): 209-214.
- 24. Tornvall, P., Olivecrona, G., Karpe, F., et al. Lipoprotein lipase mass and activity in plasma and their increase after heparin are separate parameters with different relations to plasma lipoproteins. (1995) Arterioscler Thromb Vasc Biol 15(8): 1086-1093.
- 25. Vilella, E., Joven, J., Fernández, M., et al. Lipoprotein lipase in human plasma is manly inactive and associated with cholesterol-rich lipoproteins. (1993) J Lipid Res 34(9): 1555-1564.
- 26. Karpe, F., Olivecrona, T., Olivecrona, G., et al. Lipoprotein lipase transport in plasma: role of muscle and adipose tissues in regulation of plasma lipoprotein lipase concentrations. (1998) J Lip Res 39(12): 2387-2393.
- 27. Casanovas, A., Parramon, N., de la Cruz, F., et al. Retroperitoneal white adipose tissue lipoprotein lipase activity is rapidly down-regulated in response to acute stress. (2007) J Lipid Res 48(4): 863-868.
- 28. Henderson, A.D., Richmond, W., Elkeles, R.S. Hepatic and lipoprotein lipases selectively assayed in postheparin plasma. (1993) Clin Chem 39(2): 218-223.
PubMed||CrossRef||Others
- 29. Donahoo, W.T., Jensen, D.R., Shepard, T.Y., et al. Seasonal variation in lipoprotein lipase and plasma lipids in physically active, normal weight humans. (2000) J Clin Endocrinol Metab 85(9): 3065-3068.
- 30. Gordon, D.J., Trost, D.C., Hyde, J., et al. Seasonal cholesterol cycles: the Lipid Research Clinics Coronary Primary Prevention Trial placebo group. (1987) Circulation 76(6): 1224-1231.
- 31. Dark, J., Zucker, I., Wade, G.N. Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. (1983) Am J Physiol 245: R334-338.
- 32. Bartness, T.J., Goldman, B.D. Peak duration of serum melatonin and short-day responses in adult Siberian hamsters. (1988) Am J Physiol 255: R812-822.
- 33. Sabugal, R., Robert, M.Q., Julve, J., et al. Hepatic regeneration induces changes in lipoprotein lipase activity in several tissues and its re-expression in the liver. ((1996) Biochem J 318: 597-602.
- 34. Chajek-Shaul, T., Friedman, G., Stein, O., et al. Endogenous plasma lipoprotein lipase activity in fed and fasting rats may reflect the functional pool of endothelial lipoprotein lipase. (1985) Biochim Biophys Acta 837: 271-278.
- 35. Hamilton, M.T., Etienne, J., McClure, W.C., et al. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. (1998) Am J Physiol 275: E1016-1022.
- 36. Palmero, E., Ricart, D., Llobera, M., et al. Partial hepatectomy and/or surgical stress provoke changes in the expression of lipoprotein lipase and actin in liver and extrahepatic tissues. (1999) Biochim Biophys Acta 1141: 61-68.
- 37. Arasaradnam, M.P., Morgan, L., Wright, J., et al. Diurnal variation in lipoprotein lipase activity. (2002) Ann Clin Biochem 39: 136-139.
- 38. Simmonds, M.J., Baskurt, O.K., Meiselman, H.J., et al. A comparison of capillary and venous blood sampling methods for the use in haemorheology studies. (2011) Clin Hemorheol Microcirc 47: 111-119.
- 39. Green, B.P., Gonzalez, J.T., Thomas, K., et al. Agreement between fingertip-capillary and antecubital-venous appetite-related peptides. (2014) Endocr Connect 3: 233-242.
- 40. Boyd, R., Leigh, B., Stuart, P. Capillary versus venous bedside blood glucose estimations. (2005) Emerg Med J 22: 177-179.
- 41. Tsutsumi, K. Lipoprotein Lipase and Atherosclerosis. (2003) Current Vascular Pharmacology 1: 11-17.
PubMed||CrossRef||Others
- 42. McNally, D., Matheson, L.A., Sankaran, K., et al. Capillary blood sampling as an alternative to venipuncture in the assessment of serum 25 hydroxyvitamin D levels. (2008) J Steroid Biochem Mol Biol 112: 164-168