Design, Formulation and Evaluation of Extended Release Tablet Containing Nisoldipine
Ghodgaonkar.SA, Vilegave.KV
Affiliation
1Shivgita Institute of Pharmacy, Asangaon-421601
2Shivajirao.S. Jondhle College of Pharmacy, Asangaon-421601
Corresponding Author
Ganeshmal Chaudhari, Shivgita Institute of Pharmacy, Asangaon, India. Email: ganeshmalchaudhari@gmail.com
Citation
Chaudhari, G.D., et al. Design, Formulation and Evaluation of Extended Release Tablet Containing Nisoldipine (2019) J pharma pharmaceutics 6(1): 1- 9.
Copy rights
© 2018 Chaudhari, G.D. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Nisoldipine; Extended release tablets; Zero order kinetics; Stability testing
Abstract
Sulfamethoxazole is a broad spectrum antibiotic that acts as a bacteriostatic antibacterial agent. This study was aimed to analyze the effect of Consciousness Energy Healing Treatment (the Trivedi Effect®) on the physicochemical and thermal properties of sulfamethoxazole with the help of advanced analytical techniques. Sulfamethoxazole sample was divided into two parts. Among these, one part received Consciousness Energy Healing Treatment remotely by a well-known Biofield Energy Healer, Alice Branton and termed as the treated sample. While the other part did not receive the Consciousness Energy Healing Treatment called a control sample. The study revealed that the particle size values in the treated sample were significantly reduced by 19.71% (d10), 6.28% (d50), 5.40% (d90), and 7.98% {D (4, 3)}; thus, the specific surface area was significantly increased by 12.63% compared with the untreated sample. The powder XRD peak intensities and crystallite sizes were altered significantly ranging from -32.49% to 120.17% and 0.45% to 101.86%, respectively; whereas, the average crystallite size was significantly increased by 33.13% in the treated sample compared with the control sample. The TGA data reported 8.35% increase in total weight loss and 19.55% significant reduction of the residual amount in the treated sample compared with the control sample. The latent heat of fusion was significantly increased by 10.34% and the degradation temperature along with the corresponding latent heat of decomposition of the treated sample was increased by 5.52% and 11.16%, respectively compared with the untreated sample. The Trivedi Effect®-Consciousness Energy Healing Treatment might have created a new polymorphic form of sulfamethoxazole which would be better soluble and bioavailable compared with the untreated sample. The Trivedi Effect Treated sulfamethoxazole might be more beneficial for designing more efficacious novel nutraceutical / pharmaceutical formulations for the prevention and treatment of various diseases such as urinary tract infections, ear infections, traveler’s diarrhea, shigellosis, bronchitis, and Pneumocystis jiroveci pneumonia, etc.
Introduction
Oral drug delivery is the most convenient and preferable route of drug administration considering patient compliance, low cost, flexibility in drug design and ease of production[1-10]. Extended release matrix tablets are relatively simple systems that are more flexible in terms of variations in ingredients, production methods, and end-use conditions than film coated ER tablets and other systems. This results in more uniform release profiles with a high resistance to dose dumping[11,12]. The most common approach of extending and controlling rate of release of drug is to incorporate a drug in hydrophilic colloid matrix such as hydroxyl propyl methyl cellulose[13]. Currently available therapies for hypertension include drugs which block the activity of peripheral sympathetic nervous system, diuretics, centrally acting drugs, selective and non-selective α and β receptor blockers, calcium channel blockers, vasodilators, ACE inhibitors, angiotensin receptor blockers, used individually or in combination[14-20]. Most of these drugs have different kinds of disadvantages associated with them such as short half-life, low bioavailability, poor permeability and adverse effects associated with them. Various attempts have been made to design drug delivery systems for calcium channel blockers to:
• reduce the dosing frequency,
• increase the bioavailability and decrease the degradation/metabolism in the gastrointestinal tract,
• improve the central nervous system (CNS) penetration and inhibit the CNS efflux, and
• Deliver them to the target cells selectively with minimal side.
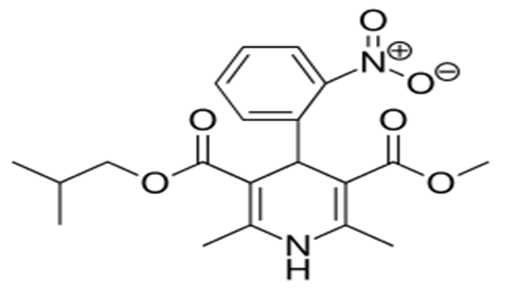
Nislodipine is an extended release tablet dosage form of the dihydropyridine calcium channel blocker. Nisoldipine is 3, 5-pyridinedicarboxylic acid, 1, 4-dihydro-2, and 6-dimethyl-4-(2-nitrophenyl)-, methyl-2-methylpropyl ester, C20H24N2O6, and has the structural formula:
Nisoldipine belongs to the class of dihydropyridine class of calcium channel blockers (calcium channel inhibitors or slow channel blockers) that prevents the transmembrane influx of calcium into vascular smooth muscle and cardiac muscle. It reversibly competes with other dihydropyridines for binding to the calcium channel. The heart contracts as a result of movement of calcium ions through specific ion channels. Blockage of such ion channels causes dilation of arterioles. In vitro study profile of nisoldipine indicates that contractile processes are selective, with greater potency on vascular smooth muscle than on cardiac muscle. Although, like other dihydropyridine calcium channel blockers, nisoldipine has negative inotropic affects as justified in vitro, studies. The effect of nisoldipine on blood pressure is principally a consequence of a dose-related decrease of peripheral vascular resistance. Nisoldipine exhibits a mild diuretic effect[21].
Materials and Methods
Physical properties of nislodipine such as bulk density tap density and powder compressibility were evaluated. The results are as given in Table 1.
Table 1: Physical properties of Nislodipine.
| Ingredient | Bulk Density | Tapped Density | Hausner ratio | Compressibility Index | Angle of Repose |
|---|---|---|---|---|---|
| API | 0.42 | 0.59 | 1.40 | 40.47 | 490 |
Particle size determine by Malvern method: Particle size is one of the most important properties influencing the dissolution rate of a drug and thus potentially its bioavailability. Particle size reduction (e.g. micronization) is often utilized to enhance dissolution rate. Small particles present a larger surface area per unit length to the dissolution media and hence dissolve more rapidly than large particles. Particle size may also affect the stability of drug substances, in that it governs the surface area available for oxidation and hydrolysis. Surface area is critical for interaction with excipients in tablet dosage forms and can greatly affect stability[22].
Results
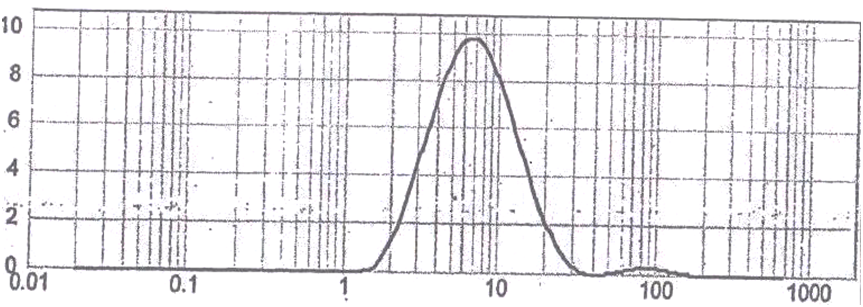
Active Pharmaceutical Ingredients (API) complies as per the specification 90 % having particle size 15.14 micron as seen in Figure 1.
Figure 2: Particle Size Distribution.
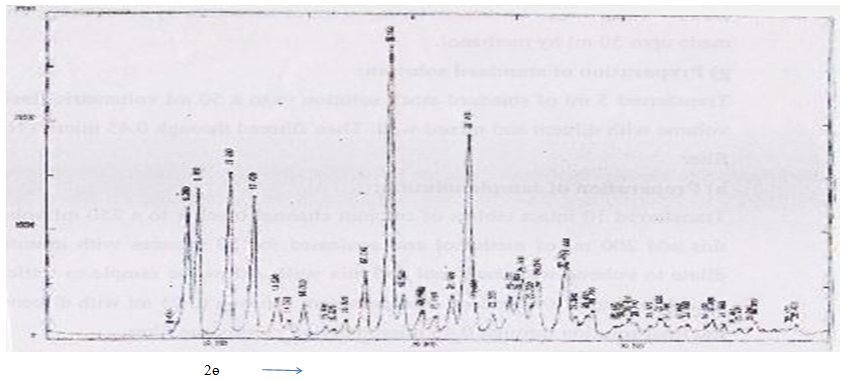
X-Ray Diffraction Studies: The X-Ray diffraction pattern of candidate drug exhibited sharp, highly intense and less diffused peaks indicating the crystalline nature of drug.
Figure 3: XRD analysis of candidate drug.
Analytical method
Instrumentation: Water Alliance HPLC system or equivalent.
Preparation of buffer: Add 1.36 gm of potassium dihydrogen orthophosphate and dissolve in 1000 ml of water, mix well and filter through 0.45 micron size membrane filter.
Preparation of mobile phase: Mixture of buffer and methanol is taken in the ratio of 32:68 and degased.
Preparation of diluents: Mixture of water and methanol is taken in the ratio of 50:50 and degased.
Chromatographic conditions: Column: Graces Kromasil C 18 (150 x 4.6 mm), 5 micron is selected.
Flow: 1.7 ml / min
Wavelength: 237 nm
Injection volume: 10 micron
Preparation of standard stock solution: Precisely 41 mg of drugs were dissolved in 40 ml of methanol by sonication. Then volume was made up to 50 ml by methanol.
Preparation of standard solution: Transfer 5 ml of standard stock solution in to a 50 ml volumetric flask and make up the volume with diluents and mix well. Then filter through 0.45 micron nylon membrane filter.
Preparation of sample solution: Transfer 10 intact tablets of calcium channel blocker in a 250 ml volumetric flask, to this add 200 ml of methanol and sonicate for 30 min with intermittent shaking. Make up the volume with methanol and mix well. Allow the sample to settle down for more than 15 minutes. Dilute 3 ml of supernatant solution to 25 ml with diluents and mix. Filter the solution through 0.45 micron nylon membrane filter.
Procedure: Inject diluents as blank, standard solution and sample solution into chromatographic system. Measure the peaks area count for candidate drug.
Formula: % Assay of candidate drug for 17 mg strength.

Where
AT: average peak area counts of drug in sample solution
AS: average area counts of drug in standard solution
W: Weight of drug working standard in mg
N: Number of tablet taken for analysis
P: Percent potency of drug
L: Label claim of drug candidate in mg per tablets
Discussion
Assay of API on the dried basis = 99.7 %
Table. 2: Determination of solubility.
| pH range | Solubility (mcg/ml) |
|---|---|
| Distilled water | 1.1394 |
| Phosphate buffer pH 2 | 1.2500 |
| Phosphate buffer pH 4 | 1.2175 |
| Phosphate buffer pH 6 | 1.2983 |
| Phosphate buffer pH 6.8 | 1.0400 |
| Phosphate buffer pH 8 | 0.7463 |
| SLS 0.25% | 24.3 |
| SLS 0.50% | 48.4 |
API (CCB) showed more solubility in SLS containing media, its solubility increases as the concentration of SLS increased.
API showed minimum solubility in water as well as in phosphate buffer without SLS at different ranges of pH.
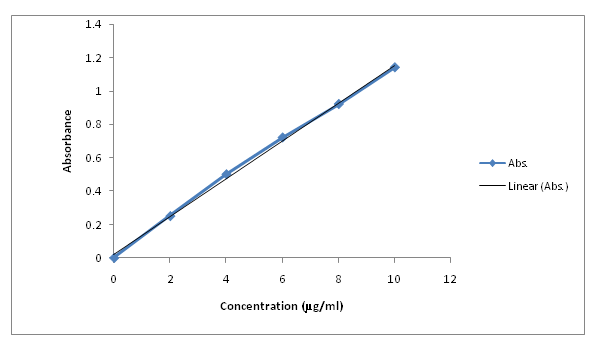
Calibration curve of API: The following concentrations were taken and the absorbance for respective concentrations was obtained. A calibration plot was obtained and the equation obtained. The equation was as follows Y = 0.113x + 0.022. The value of R2 was found to be 0.998. On the basis of results obtained it was concluded that Active Pharmaceutical Ingredient (API), obeyed Beer – Lambert’s law in the range of 2- μg / ml.
Table. 3: Concentration of standard solutions and their absorbance.
| Sr. No. | Conc (μg/ml) | Absorbance |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 2 | 0.25 |
| 3 | 4 | 0.50 |
| 4 | 6 | 0.72 |
| 5 | 8 | 0.92 |
| 6 | 10 | 0.139 |
Figure 4: Calibration curve of active pharmaceutical ingredient.
Drug-Polymer Interactions: Physical observation of drug excipients compatibility study at accelerated condition (40°C / 75 % RH).
Table 4: Physical observation of drug excipients compatibility study at accelerated condition (40°C/75% RH).
| Sr No | Physical admixture | Drug excipient ratio | Initial description | Observation |
|---|---|---|---|---|
| 1 | API | ......... | Yellow powder | NC |
| 2 | API+ Methocel K100LVCR | 1:17 | Light yellow powder | NC |
| 3 | API+MethocelK4MCR | 1:5 | Light yellow powder | NC |
| 4 | API + Hydroxypropyl Cellulose (HPC-L) | 1:5 | Yellow powder | NC |
| 5 | API + Hydroxypropyl cellulose (HPC-M) | 1:5 | Yellow powder | NC |
| 6 | API +Lactose Monohydrate NF | 1:7 | Light yellow powder | NC |
| 7 | API + Sodium lauryl sulphate | 1:3:5 | Light yellow powder | NC |
| 8 | API + Eudragit L30D-55 | 1:2 | Light yellow powder | NC |
| 9 | API + Glyceryl behente NF (Compritol888AT) | 1:1 | yellow powder | NC |
| 10 | API + Collodial silicon dioxide NF (Aerosil200) | 1:1 | yellow powder | NC |
| 11 | API + Magnesium Sterate NF | 1:0:5 | yellow powder | NC |
| 12 | API + Methocel E50 | 1:17 | Light yellow powder | NC |
| 13 | Opadry 03f52057 | 1:5 | Light yellow powder | NC |
| 14 | API + Methocel K100M CR | 1:1:7 | Light brown powder | NC |
NC-No chang
Table 5: Formulation of tablets.
| Sr. no | Ingredient | T1 (Mg) | T2 (Mg) | T3 (Mg) | T4 (Mg) | T5 (Mg) | T6 (Mg) | T7 (Mg) | T8 (Mg) | T9 (Mg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CCB | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 |
| 2 | Hypromellose | 362 | 362 | 222 | 242 | 272 | 272 | 172 | 232 | 232 |
| 3 | Methocel | 100 | 100 | 50 | 30 | ---- | --- | --- | --- | |
| 4 | K100MCR Lactose monohydrate | 205 | 205 | 105 | 102 | 95 | 105 | 105 | 105 | --- |
| 5 | Povidone | 10 | 10 | 16 | 6 | 6 | 6 | 6 | 6 | 6 |
| 6 | SLS | 50 | 50 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| 7 | IPA:DCM(60:40) | -- | --- | --- | --- | --- | --- | --- | --- | --- |
| 8 | Hypromellose | 12 | 12 | --- | --- | --- | --- | --- | --- | --- |
| 9 | Eudragit | 12 | 12 | --- | --- | --- | --- | --- | --- | --- |
| 10 | Glyceryl behenate | 20 | 20 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 11 | Colloidal silicone dioxide | 8 | 8 | 6 | 6 | 6 | 7 | 7 | 7 | 7 |
| 12 | Magnesium sterate | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 |
| 13 | Opadry | --- | 24 | --- | 14 | 14 | 11 | 12.9 | 12.9 | 12.9 |
| 14 | Purified water | --- | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
| Total weight | 800 | 824 | 870 | 954 | 954 | 751 | 751 | 812 | 812 |
Table 6: Evaluation of tablets.
| Formulations | Hardness (kg/cm2 ± S.D) | Friability (%w/w) ± S.D | Thickness (mm ± S.D) | Average wt. (mg) | Film coating |
|---|---|---|---|---|---|
| T1 | 110 | Nil | 6.08 | 795 | - |
| T 2 | 106 | Nil | 6.02 | 800 | 3.21 |
| T 3 | 70 | Nil | 5.29 | 475 | 3.12 |
| T 4 | 76 | Nil | 4.80 | 473 | 3.15 |
| T 5 | 65 | Nil | 4.84 | 470 | 3.04 |
| T6 | 62 | Nil | 4.35 | 472 | 3.00 |
| T7 | 66 | Nil | 4.81 | 372 | 3.04 |
| T8 | 69 | Nil | 4.59 | 430 | 3.00 |
| T 9 | 68 | Nil | 4.42 | 432 | 3.01 |
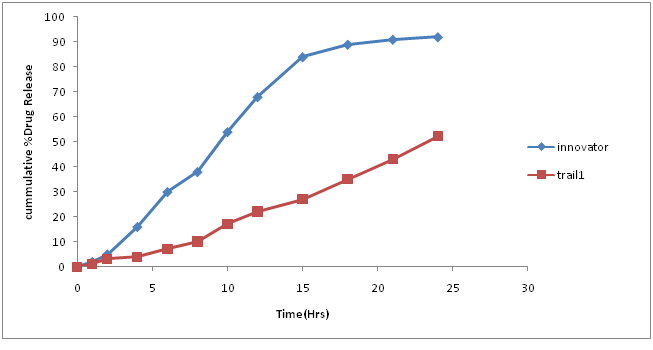
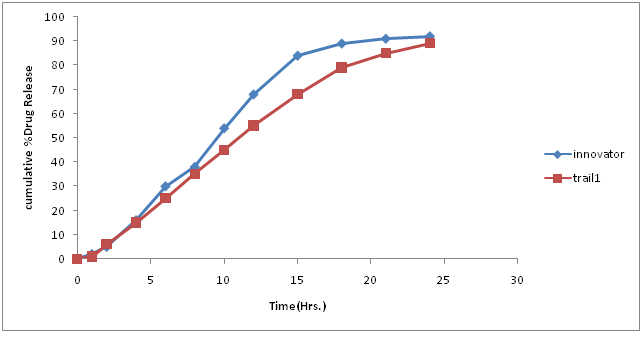
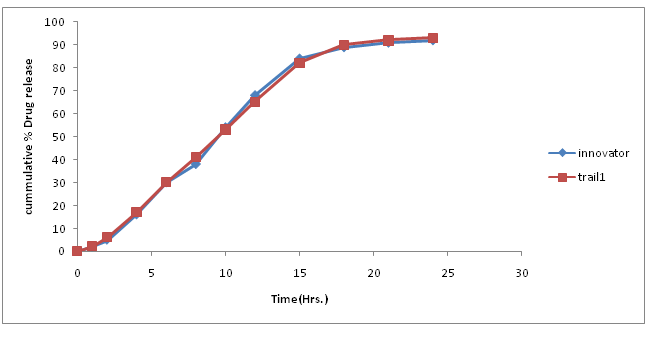
Table.7: % Drug concentration of innovator and trial batch-1.
| Time in hors | Innovator | Trial 1 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 1 |
| 2 | 5 | 3 |
| 4 | 16 | 4 |
| 6 | 30 | 7 |
| 8 | 38 | 10 |
| 10 | 54 | 17 |
| 12 | 68 | 22 |
| 15 | 84 | 27 |
| 18 | 89 | 35 |
| 21 | 91 | 43 |
| 24 | 92 | 52 |
| F2 | 17.15 |
Figure 5: Dissolution curve of Trial-1 batch.
Discussion of Trial number 1
• Current batch showed formation of fine (>70%) and poor flow property, which is unacceptable for compression process. Hence change in granulation process is required to increases the amount of granules.
• Dissolution profile was very slow as compare to innovator dissolution profile.
• Change in granulation process was decided to minimize fine formation.
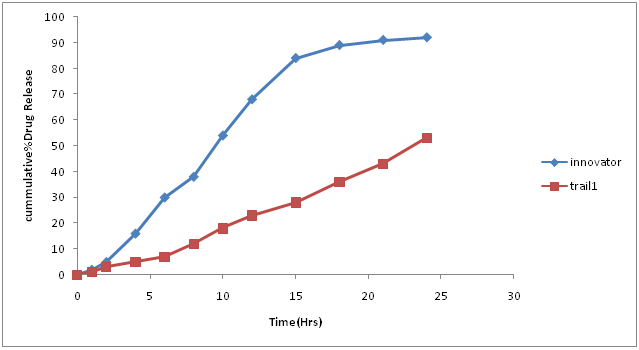
Table. 8: % Drug concentration of innovator and trial batch-2.
| Time in hors | Innovator | Trial 2 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 1 |
| 2 | 5 | 3 |
| 4 | 16 | 5 |
| 6 | 30 | 7 |
| 8 | 38 | 12 |
| 10 | 54 | 18 |
| 12 | 68 | 23 |
| 15 | 84 | 28 |
| 18 | 89 | 36 |
| 21 | 91 | 43 |
| 24 | 92 | 53 |
| F2 | 17.51 |
Figure 6: Dissolution curve of Trial-2 batch.
Discussion of Trial no-2: Quantity of fine flowing powder when decreased causes slowing of the drug release profile is as compared to innovator profile so the next batch plan begins with the increase in amount of sodium lauryl sulphate to increases the rate and extend of drug from tablet.
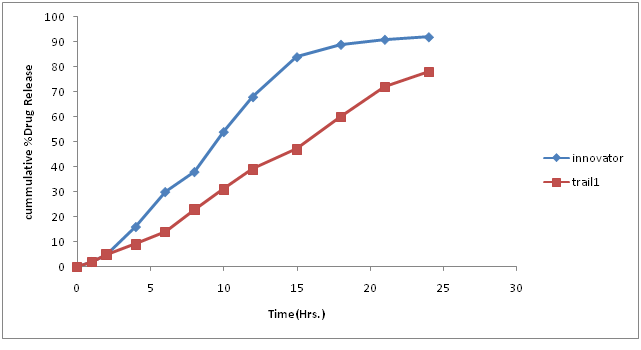
Table 9: % Drug concentration of innovator and trial batch-3.
| Time in hours | Innovator | Trial 3 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 2 |
| 2 | 5 | 5 |
| 4 | 16 | 9 |
| 6 | 30 | 14 |
| 8 | 38 | 23 |
| 10 | 54 | 31 |
| 12 | 68 | 39 |
| 15 | 84 | 47 |
| 18 | 89 | 60 |
| 21 | 91 | 72 |
| 24 | 92 | 78 |
| F2 | 27.01 |
Figure 7: Dissolution curve of Trial-3 batch.
Granulation and compression parameters were satisfactory.
Drug release was slower compare to innovator profile. Hence composition should be changed Next batch was planned with decreased quantity of methocel K100MCR. Because it is a high viscosity grade polymer which slows down the dissolution rate as it is controlled release polymer. Therefore by decreasing quantity of his polymer there may be chances of increases in drug release profile.
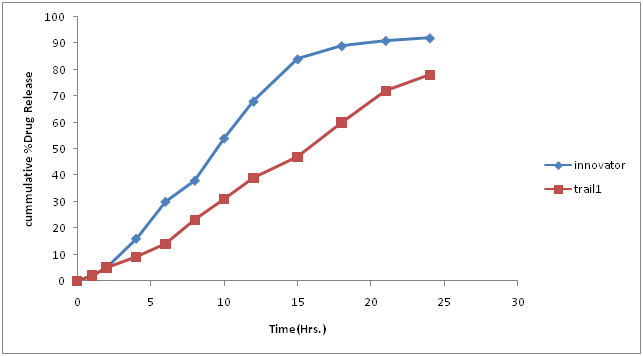
Table 10: % Drug concentration of innovator and trial batch-4.
| Time in hours | Innovator | Trial 3 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 1 |
| 2 | 5 | 4 |
| 4 | 16 | 11 |
| 6 | 30 | 19 |
| 8 | 38 | 28 |
| 10 | 54 | 39 |
| 12 | 68 | 48 |
| 15 | 84 | 61 |
| 18 | 89 | 73 |
| 21 | 91 | 82 |
| 24 | 92 | 85 |
| F2 | 33.97 |
Figure 8: Dissolution curve of Trial-4 batch.
Discussion of Trial no 4
• Compression parameter was satisfactory and was similar to trial number 3.
• It was found that drug release profile is still slow and not matching with innovator profile.
• So in the next batch it is planned that Eudragit L30 D-55 would be added for granulation and methocel K100L VCR as matrix former.
Table 11: % Drug concentration of innovator and trial batch-5.
| Time in hours | Innovator | Trial 5 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 1 |
| 2 | 5 | 6 |
| 4 | 16 | 15 |
| 6 | 30 | 25 |
| 8 | 38 | 35 |
| 10 | 54 | 45 |
| 12 | 68 | 55 |
| 15 | 84 | 68 |
| 18 | 89 | 79 |
| 21 | 91 | 85 |
| 24 | 92 | 89 |
| F2 | 39.90 |
Figure 9: Dissolution curve of Trial-5 batch.
Discussion of Trial number 5
• Drug release profile was somewhat similar to innovator at starting phase but later it slowed down.
• Hence it was thought decided that active pharmaceutical ingredients (API) of size less than 10 microns can be used to enhance the drug release profile.
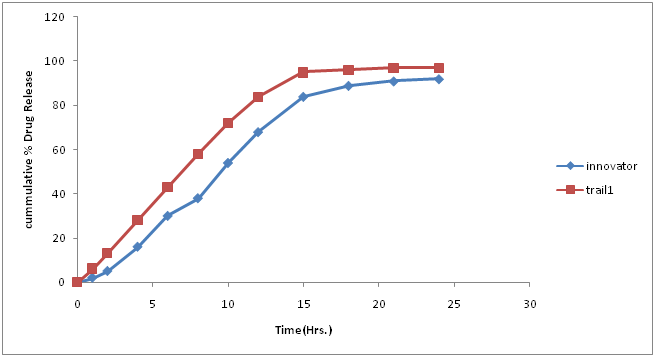
Table 12: % Drug concentration of innovator and trial batch-6.
| Time in hours | Innovator | Trial 5 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 6 |
| 2 | 5 | 13 |
| 4 | 16 | 28 |
| 6 | 30 | 43 |
| 8 | 38 | 58 |
| 10 | 54 | 72 |
| 12 | 68 | 84 |
| 15 | 84 | 95 |
| 18 | 89 | 96 |
| 21 | 91 | 97 |
| 24 | 92 | 97 |
| F2 | 46.76 |
Figure 10: Dissolution curve of Trial-6 batch.
Discussion of Trial number 6
• Drug release profile was faster at initial phase but latter it’s showed similar drug release as compared to innovator profile.
• It was further thought to increase the quantity of methocel E-50 to match drug release profile with innovator profile.
Table 13: % Drug concentration of innovator and trial batch-7.
| Time in hours | Innovator | Trial 7 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 4 |
| 2 | 5 | 9 |
| 4 | 16 | 22 |
| 6 | 30 | 34 |
| 8 | 38 | 46 |
| 10 | 54 | 60 |
| 12 | 68 | 73 |
| 15 | 84 | 88 |
| 18 | 89 | 89 |
| 21 | 91 | 91 |
| 24 | 92 | 93 |
| F2 | 67.23 |
Figure 11: Dissolution curve of Trial-7 batch.
Discussion of Trial number 7
• Drug release profile was faster at initial phase but latter it’s showed similar drug release as compared to innovator profile.
• Hence it was planned to increase the quantity of methocel E-50 to match drug release profile with innovator profile.
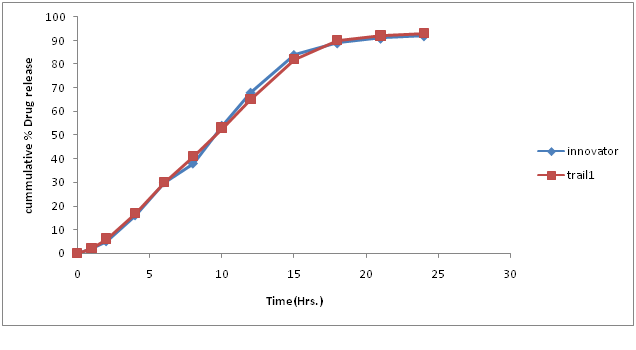
Table 14: % Drug concentration of innovator and trial batch-8.
| Time in hours | Innovator | Trial 7 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 2 |
| 2 | 5 | 7 |
| 4 | 16 | 18 |
| 6 | 30 | 30 |
| 8 | 38 | 42 |
| 10 | 54 | 53 |
| 12 | 68 | 64 |
| 15 | 84 | 81 |
| 18 | 89 | 89 |
| 21 | 91 | 94 |
| 24 | 92 | 94 |
| F2 | 80 |
Figure 12: Dissolution curve of Trial-8 batch.
Discussion of Trial number 8
• Drug release profile of test batch was close to drug release profile of innovator in official media; F2 value was 79.30.
• Compression parameter was satisfactory found in this batch.
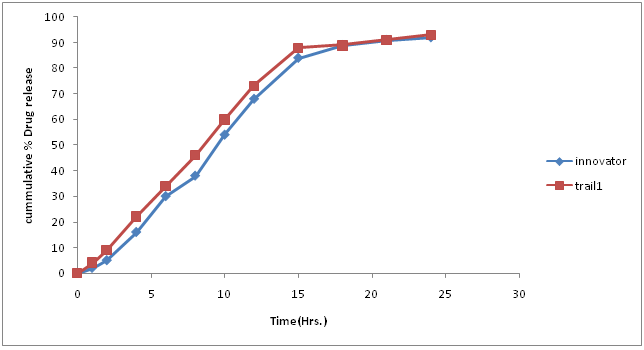
Table 15: % Drug concentration of innovator and trial batch-9.
| Time in hours | Innovator | Trial 9 |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 2 | 2 |
| 2 | 5 | 6 |
| 4 | 16 | 17 |
| 6 | 30 | 30 |
| 8 | 38 | 41 |
| 10 | 54 | 53 |
| 12 | 68 | 65 |
| 15 | 84 | 82 |
| 18 | 89 | 90 |
| 21 | 91 | 92 |
| 24 | 92 | 93 |
| F2 | 86.92 |
Figure 13: Dissolution curve of Trial-9 batch.
Discussion of Trial number-9
• Drug release profile of test batch was close to drug release profile of innovator in official media; F2 value was 86.92.
• Compression parameter was satisfactory found in this batch
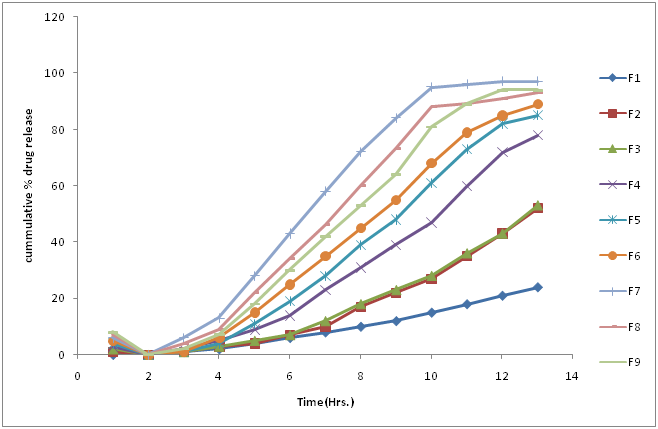
Table 16: Zero order release kinetics result.
| Time points | Initial (%) | 1 month (%) | 2 month (%) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 2 | 2 | 2 |
| 2 | 7 | 6 | 6 |
| 4 | 18 | 18 | 17 |
| 6 | 30 | 29 | 28 |
| 8 | 42 | 40 | 40 |
| 10 | 53 | 52 | 50 |
| 12 | 64 | 62 | 62 |
| 15 | 81 | 79 | 79 |
| 18 | 89 | 89 | 87 |
| 21 | 94 | 92 | 93 |
| 24 | 94 | 93 | 93 |
Figure 14: Zero order release of all trial batches.
Conclusion
In the given Trial batches dissolution release (Trial number 8) is release by zero order kinetics i.e. the constant amount of release dose does not depend of drug concentration[23].
Table 17: Assay result.
| Sr.no | Trial 1 | Trial 2 | Trial 3 | Trial 4 | Trial 5 | Trial 6 | Trial 7 | Trial 8 | Trial 9 |
|---|---|---|---|---|---|---|---|---|---|
| Assay % w/w | 98.60 | 98.23 | 97.86 | 99.23 | 98.66 | 100.86 | 97.23 | 100.6 | 99.56 |
Table 18: Stability data.
| Parameter | Initial | Months & condition 40°C/75 % RH | |
|---|---|---|---|
| 1M | 2M | ||
| Assay (%w/w)(By HPLC) | 100.6 | 98.9 | 99.5 |
Conclusion
• The drug excipients compatibility studies showed that the studied excipients have no interaction with drug. the excipients were compatible with API.
• Evaluation of physiochemical parameter like hardness, friability, dissolution and assay indicated that the tablet were mechanically stable and complied with necessary pharmacopoeia specification and comparable to innovator product.
• The stability testing of finalized batch at 40°C / 75 % RH revealed no significant change with respect to assay and drug release pattern that indicate the stability of the finished product.
• Finally, it is concluded that the process adopted for the manufacturing provides a product meeting all the predetermined specification and quality characteristics. The process would imbibe reproducibility and robustness in the formulation.
Future Scope
• The research project has a wide future scope to modify the delivery system of drug for the treatment of angina.
• Following are the certain future scope;
• Bioequivalent study.
• Development of other strength using scale up /scale down technique.
• ANDA filing
References
- 1. Tiwari,S.B., Rajabi-Siahboomi, A.R. Modulation of drug release from hydrophilic matrices. (2008) Pharm Tech com 20(9).
Pubmed|| Crossref|| Others
- 2. Using Methocel Cellulose Ethers for Controlled Release of Drugs in Hydrophilic Matrix Systems.
Pubmed|| Crossref|| Others
- 3. Pradhan, R., Budhathoki, U., Thapa, P. Formulation of once a day controlledrelease tablet of indomethacin based on HPMC mannitol. (2008) Kathmandu University Sci Eng Tech 4(1): 55-67.
- 4. Birkenhager, W.H., Reid, J.L. Pharmacology of antihypertensive drugs, Handbook of Hypertension. (1984) Elsevier 3: 1-5.
Pubmed|| Crossref|| Others
- 5. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020356s027lbl.pdf
Pubmed|| Crossref|| Others
- 6. Ansel, C.H., Poppovich, N.G. Pharmaceutical dosage forms and drug delivery systems. (2014) Lippincot Williams and Wilkins: 213.
Pubmed|| Crossref|| Others
- 7. Gupta, P.K., Robinson, J.R. Oral controlled released delivery. (1992) Treatise on controlled drug delivery, K: 255.
Pubmed|| Crossref|| Others
- 8. Chien, Y.W. Novel drug delivery system. (1992) Revised and expanded 1(2): 139-140.
Pubmed|| Crossref|| Others
- 9. Advanced drug delivery system: New development new technologies. (2009)
Pubmed|| Crossref|| Others
- 10. Longer, M.R., Robinson, J.R. Sustained-release drug delivery system. (2000) Sci Practice Pharm 1645-1650.
Pubmed|| Crossref|| Others
- 11. Brahmankar, D.M. Jaywalk, S.B. Biopharmaceutics and Pharmacokinetics-A Treatise. (1995) 347-352.
Pubmed|| Crossref|| Others
- 12. Brahmankar, D.M., Jaywalk, S.B. Biopharmaceutics and Pharmacokinetics-A Treatise. (1995) 399 - 401.
Pubmed|| Crossref|| Others
- 13. Lee, V.H., Robinson, J.R. Sustained and Controlled Release Drug Delivery system. Marcel Dekker, New York: 71-121.
Pubmed|| Crossref|| Others
- 14. Lee, V.H., Robinson, J.R. Sustained and Controlled Release Drug Delivery system, Marcel Dekker, New York: 138-171.
Pubmed|| Crossref|| Others
- 15. Sansom, L. N. Oral extended Release product. (1999) Australian Prescriber 88-90.
Pubmed|| Crossref|| Others
- 16. Lachman, L., Liberman, H.A., Kanig, J.L. The theory and Practice of Industrial Pharmacy. (1986) Varghese Publishing House Bombay.
Pubmed|| Crossref|| Others
- 17. Chain, Y.W. Novel drug delivery system. (1983) Marcel Dekker Inc New York. 141-193.
Pubmed|| Crossref|| Others
- 18. Robinson, G.M. Sustained and Controlled Release Drug delivery System. 376-417.
Pubmed|| Crossref|| Others
- 19. Obaidat, A.A., Obaidat, R.M. Controlled release of tramadol hydrochloride from matrices prepared using glycerylbehanate. (2001) Eur J Pharm Biopharm 52(2): 231-235.
- 20. Makhija, S.N., Vavia, P.R. Once daily sustained release tablets of venlafaxine, an ovel antidepressant. (2002) Eur J Pharm Biopharm 54(1): 9-15.
- 21. Ceballos, A., Cirri. M.F., Corti, G., et al. Influence of formulation and process variables on in vitro release of theophylline on directly compressed Eudragit Matrix tablets. (2005) Farmaco 60(12): 913-918.
- 22. Patel, R., Baria, A. Formulation development and process optimization of theophylline sustained release matrix tablet. (2009) Int J Pharm pharmaceutical sci 1(2): 30-42.
Pubmed|| Crossref|| Others
- 23. Kathiresen, K., Kiran, K.V., ijin, P., et al. Formulation development of indomethacin sustained release matrix tablet. (2010) J Pharmtech Res 2(1): 794-797.
Pubmed|| Crossref|| Others