Drug Utilization Review of Fluoroquinolones in Outpatient Department of Nekemte Specialized Hospital, Western Ethiopia
Ginenus Fekadu1*, Dinka Dugassa2, Firomsa Bekele3
Affiliation
1Department of Pharmacy, Institute of Health Sciences, Wollega University, Nekemte, Ethiopia
2Department of Pharmacy, Nekemte Specialized Hospital, Nekemte, Ethiopia
3Department of Pharmacy, College of Health Science, Mettu University, Mettu, Ethiopia
Corresponding Author
Ginenus Fekadu, Clinical pharmacy unit, Department of Pharmacy, Institute of Health Sciences, Wollega University, Nekemte, Oromia, Ethiopia, Fax: +251(0)576617980, Tel: +251(0)917137145 / +251(0)917733383; E-mail: take828pharm@gmail.com / ginenus@wollegauniversity.edu.et
Citation
Fekadu, G., et al. Drug Utilization Review of Fluoroquinolones in Outpatient Department of Nekemte specialized Hospital, Western Ethiopia. (2019) J pharma pharmaceutics 6(1): 40-43.
Copy rights
© 2019 Fekadu, G. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Drug use evaluation; Resistance; Irrational use of drugs; Fluoroquinolones; Nekemte; Ethiopia
Abstract
Fluoroquinolones are extremely useful agents and an important therapeutic advance. Overuse of the fluoroquinolones particularly in the hospital settings has led to selection of resistance. This resistance has increased after introduction of this class of drugs into clinical practice especially in Pseudomonas and Staphylococci. A retrospective cross-sectional study was conducted to evaluate drug use evaluation of fluoroquinolones at Nekemte Specialized Hospital (NSH). About 366 samples were taken from February 2018 to 2019 using systematic random sampling technique. Among 366 patients on fluoroquinolones, 171 (46.7%) were males and 273 (74.6%) were in the age group of 15-65 years. It was found that 366(100%) of ciprofloxacin doses and frequencies were correct while 92% of the duration was correct. From fluoroquinolones prescribed with incorrect durations, about 24(6.55%) were prescribed for longer duration and 2(0.5%) were given with short duration. It was found that 25.8% of fluoroquinolones had one or more potential interaction particularly with diclofenac (22.6%). Fluoroquinolones were used with contra indication in 11.3% of the cases. There was a great problem concerning the duration, use along with potentially interacting drugs and against contraindications of fluoroquinolones therapy. Hence, prescribers should be adhered to updated standard treatment guidelines while prescribing drugs.
Introduction
Irrational drug use is an inappropriate use of dose, duration, indication and costly medication in which the patients are incompliant to it[1]. Antibiotics are among the most frequently prescribed medications today although microbial resistance due to evolutionary pressures and misuse threatens their continued efficacy[2-4]. The use of inappropriate a dose may result in treatment failure and is most likely to select for microbial resistance. Dosing errors which can be the wrong frequency of administration or the use of either an excessive or sub-therapeutic dose are common in using antimicrobial agents[3].
Excessive and irrational use of antimicrobial drugs is common in all countries. It is particularly troublesome in developing countries where there is a heavy burden of infectious diseases[1]. Overuse and inappropriate use of antibiotics has fueled a major increase in prevalence of multidrug resistant pathogens, leading some to speculate that we are nearing the end of the antibiotic era[4]. Resistant organisms may emerge as a result of many factors, including irrational use of drugs one form of which is irrational prescribing practice[1,5].
Fluoroquinolones are extremely useful agents and an important therapeutic advance. The introduction of fluoroquinolones represents important therapeutic advance because these agents have broad antimicrobial activity and are effective after oral administration for the treatment of a wide variety of infectious diseases[3,4]. This antimicrobial agents have paramount importance in treating infections caused by many Enterobacteriaceae and other gram negative bacilli. They are also the drugs of choice for prophylaxis and treatment of anthrax[6,7].
Overuse of the fluoroquinolones particularly in the hospital settings has led to selection of resistance and this resistance has increased after their introduction into clinical practice especially in Pseudomonas and Staphylococci[3,4]. The resistance associated with fluoroquinolones increased by two to nine folds over the era of periods[8].
One of the most pressing problems facing public health providers and administrators in many countries is ensuring rational use of drugs[9]. Rational drug use implies an individual approach to patient treatment. One mechanism to ensure correct prescribing is with drug use evaluation (DUE)[9-11]. This study was aimed to evaluate the use of fluoroquinolones at Nekemte Specialized Hospital (NSH).
Materials and Methods
Study Area: The study was conducted in Nekemte town, Oromia regional state with an estimate population of 150,000. Nekemte Specialized Hospital was the governmental hospital established by missionaries in 1934 and having a total of 338 beds. It provides general out patient, major and minor operation, inpatient, physiotherapy, pharmacy, laboratory and imaging services. A cross sectional, retrospective study was conducted from February 2018 to February, 2019. Patients Medical History Cards (PMHC) with fluoroquinolones during the study period were included.
Sampling sample size and techniques: Single population proportion formula was used to calculate the required sample size considering the following assumptions: n is required sample size, P = proportion of use of fluoroquinolones use-50%; Z = the standard normal deviation at 95% confidence interval which is 1.96; D = degree of accuracy desired marginal error which is 5%; n= the required minimum sample size.
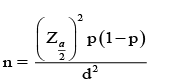
z = 1.96
P = 50 % (0.5)
D = 0.05
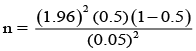 n = 384
n = 384
N = the total number of patients who have taken fluoroquinolones during study period were 7, 361. The corrected sample size using the correction formula was 366. Study participants were selected by using systematic random sampling technique.
Data collection process and analysis: The sample was taken from patients’ medical history cards. A semi-structured data collection format adopted from different literatures was used for data collection. The data was collected by two nurses from outpatient department of NSH. Appropriateness of the dosage regimens (dose, frequency and duration) of fluoroquinolones were identified based on standard guidelines of Ethiopia for treatment of different infections. Five percent of the sample was pre-tested to check acceptability and consistency of data collection tool two weeks before the actual data collection. The collected data was analyzed using SPSS v.20. The results of the data was presented using frequency tables, graphs and pi-charts.
Results
Out of a total of 366 outpatients receiving fluoroquinolones, 195 (53.2%) were females and 25 (6.8%) were pregnant mothers (Table 1).
Table 1: Sociodemographic characteristics of patients’ taking Fluoroquinolones in the Outpatient Department of NSH, from February, 2018 to 2019.
|
Variable |
Number |
Percent |
||
|
Age |
Children ( ˂ 18) |
10 |
2.7% |
|
|
Adult (18 - 65) |
273 |
74.6% |
||
|
Elder ( > 65) |
83 |
22.6% |
||
|
Total |
366 |
99.9% |
||
|
Sex |
Male |
171 |
46.7% |
|
|
Female |
Pregnant |
25 |
6.8% |
|
|
Breast feeding |
2 |
0.5% |
||
|
Others |
168 |
45.9% |
||
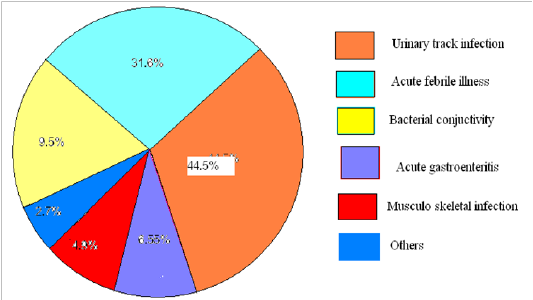
From 366 patients with fluoroquinolones, majority of the patients (44.5%) used for the treatment of urinary tract infection (UTI) and for acute febrile illness (31.6%) (figure 1).
Figure 1: Disease states for using fluoroquinolones in the outpatient department of Nekemte Specialized Hospital, February, 2018 to February, 2019.
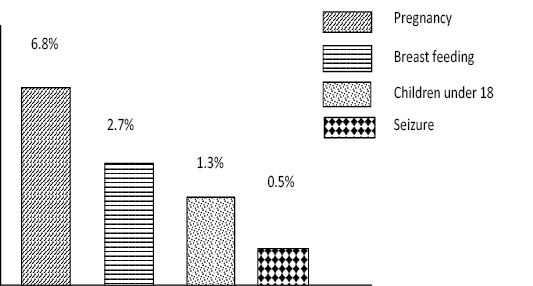
It was found that 366(100%) of ciprofloxacin dose and frequency were correct while 92% of the duration was correct. From fluoroquinolones prescribed with incorrect durations, about 24(6.55%) were prescribed for longer duration and 2(0.5%) were given with short duration. It was found that 25.8% of fluoroquinolones use had one or more potentially drug interaction particularly with diclofenac (22.6%), paracetamol (1.3%) and antacids (1.9%). Fluoroquinolones were used with contra indicated in 11.3% of the cases. There were 6.8% pregnant and 2.7% breast feeding mothers for whom fluoroquinolones were prescribed in our study finding (figure 2).
Figure 2: Use of fluoroquinolones in the presence of contraindication in the outpatient department of Nekemte Specialized Hospital February, 2018 to February, 2019.
From the total patients with fluoroquinolones, 329 (89%) were indicated for treatment based on kinetic diagnosis, 27 (7.3%) based on empirical diagnosis and 10 (2.7%) patients received as prophylaxis.
Discussion
The purpose of drug use evaluation is to ensure that drugs are used appropriately, safely and effectively for the patients. The success of treatment largely depends on the ability of healthcare providers to diagnosis the major health problem, select the correct drug, dosage form and route of administration[11-13]. Drugs have to be prescribed appropriately on the basis of clinical disease identified through diagnosis. Hence prescribes should familiar with standard treatment guidelines while prescribing prescription only drugs.
Regarding indications of fluoroquinolones, the study showed that 100% of indications were appropriate as the national drug formulary which was even a better performance as compared to the threshold set for indication of fluoroquinolones (90%). The finding was comparable with a retrospective study done in Cleveland veterans’ administration medical center which revealed that 100% of fluoroquinolones uses were appropriate[14].
Different doses of fluoroquinolones are used for variety of infections based on different age groups. Therefore, in this study 100% of fluoroquinolones doses were correctly administered. The proportion was more than the retrospective study in central North Carolina in 10 community nursing facilities which showed that only 81% of fluoroquinolones dosing was correct[15].
Drug resistances have become a great problem associated with the use of antimicrobial agents. Prolonged use of fluoroquinolones may result in microbial resistance. Hence it should be used appropriately according to the duration of treatment needed with the particular disease available in standard treatment guidelines and formularies. Inappropriate duration of therapy was the major problem revealed by this study. About 92% of fluoroquinolones use was with correct duration of therapy which was almost similar with the threshold set for fluoroquinolones duration of therapy (93%). About (0.5%) of fluoroquinolones was given with short duration while (6.5%) was given with long duration of treatment. This result showed almost similar to percentage of correct duration as comparable with previous recommended guidelines and findings[4,14].
The use of two or more drugs is recommended in specifically defined situations base on pharmacological rational. However, selection of an appropriate combination requires an understanding about potential interaction between the drugs[16]. Interactions may affect the patient negatively. From the study it was found that 25.8% of fluoroquinolones use had one or more potentially interacting with diclofenac (22.6%), paracetamol (1.3%) and antacids (1.9%). Co-administration of fluoroquinolones with analgesic may cause central nervous system toxicity and convulsion. Absorption of the fluoroquinolones may be interfered when administered with Aluminum or Magnesium containing antacids.
Prescribing drugs against contraindications should be avoided unless the benefit of doing go out weighed the risk. Fluoroquinolones were used with contraindicated in 11.3% of the cases. From these, 2.7% were children under 18 years, 6.8% pregnancy, 0.5% breast feeding mothers and 1.3% patients with seizure. Drug interaction results are different from the threshold set for drug interaction with ciprofloxacin (90%). This difference might be due to the practice of prescribing multiple drugs together[3,12,16]. On the contrary the study conducted in Borumeda hospital by Biru T et al., showed that there were no pregnant and lactating patients for whom ciprofloxacin was prescribed, but it was indicated for four (10%) children whose ages were < 18 years which was against contraindications[16]. Fluoroquinolones use along with potentially interacting drugs and against contraindications was also reported in the outpatient department of Dessie referral hospital which was similar to our study finding[17].
Conclusion
From this retrospective drug use evaluation study, it was identified that there was inappropriate fluoroquinolones use in the outpatient department of NSH even though the drug’s use regarding indications was a better performance and dosing practices were almost appropriate as per the criteria used for the study (assuming that there were no dose adjustments). There was a great problem concerning the duration of fluoroquinolones drug therapy. Fluoroquinolones use along with potentially interacting drugs and against contraindications was also another problem indicated in the study. If the appropriate drug therapy are needed to be maximized all prescribers should use up dated similar standard treatment guidelines while prescribing drugs. Regular drug use evaluation have to be undertaken in clinical setting where more drugs are prescribed to increase the patient to have or to get appropriate, safe and cost effective drug therapy.
Acknowledgement
We thank Wollega University for logistic support. We are grateful to staff members and health care professionals of NSH, data collectors and study participants for their cooperation in the success of this study.
Funding
None
Authors’ Contributions
GF contributes in the proposal preparation, study design, analysis and write up of the manuscript. FB and DD contributed to the design of the study and edition of the manuscripts. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was conducted after approval by pharmacy department of Wollega University and hospital administration of Nekemte specialized hospital. The confidentiality of patients were assured throughout by removing identifiers from data collection tools
Competing Interests
No competing interests exist.
References
- 1. Mackimite, A.R., Robertson, L., Jappy, B., et al. Audit of antibiotic policy and antimicrobial investigations for treating bactericidal in large teaching hospital. (2003) Int J Antimicrob agents 22(6): 618-621.
- 2. Lester, A. Foyes principles of Medicinal chemistry. (2002) Antibi Antimicrobial agents 5th ed: 825.
Pubmed| Crossref| Others
- 3. Henry, F., Chambers, L. B. B., Jones, L., et al. Goodman and Gilman’s pharmacologic basis of therapeutics, 11th ed: 1095 -1119.
Pubmed| Crossref| Others
- 4. Bertram, G. K. Basic and clinical pharmacology, 7th ed. (1998): 723 & 767.
Pubmed| Crossref| Others
- 5. Von, G.V., Troillet, N., Beney, J., et al. Impact of an interdisciplinary strategy on antibiotic use, A prospective controlled in three hospitals. (2005) J Antimicrob Chemother 55(3): 362-366.
- 6. Addis, A. DACA: National Drug List of Ethiopia, Drug Administration and control Authority of Ethiopia. (2004).
Pubmed| Crossref| Others
- 7. Richard, D. H., Mary J. M., Richard, A. H., et al. Lippincott’s illustrated Reviews of Pharmacology, 3rd ed. 382-383.
Pubmed| Crossref| Others
- 8. Raveh, D., Muallem Z, E., Greenberg, A., et al. Prospective drug utilization evaluation of three broad spectrum antimicrobials: Cefepime, Piperacillin- tazobactam and meropenem. (2006) OJM 99(6): 397-406.
- 9. Moore, T., Bykov, A., Savelli, T., et al. Guidelines for implementing drug utilization review programs in hospitals. (1997) Management Sci Hea, Arlington.
Pubmed| Crossref| Others
- 10. ASHP Guidelines on Medication Use Evaluation. American society of Hospital pharmacists. (1996) Am J Health- syst- Pharm 53(16): 1953-1955.
- 11. Academy of managed care pharmacy, Concepts in Managed Care pharmacy: drug use evaluation. (1998).
Pubmed| Crossref| Others
- 12. Moddley, P., Moodley, D., Sturm, A. W. Fluoroquinoles resistant Neisseria gonorrhoea in South Africa. (2004) Int J Antimicrobial Agents 24:192-193.
Pubmed| Crossref| Others
- 13. Rehana, H.S., Nagarani, M.A., Rehan, M. A study on the drug prescribing pattern and use of antimicrobial agents at a tertiary care teaching hospital in Eastern Nepal. (1998) Indian J Pharmacol 30(3): 175-180.
Pubmed| Crossref| Others
- 14. American society of hospital pharmacists: Ciprofloxacin drug utilization review and prospective drug use evaluation.
Pubmed| Crossref| Others
- 15. Richard, W. D., Todd, k. J. Edward, H. Evaluation of potentially unnecessary ciprofloxacin use in Long-Term care facilities, central North Carolina. (1999) Evaluation potentially unnecessary J 37: 21-26.
Pubmed| Crossref| Others
- 16. Tsehay, B. T., Defersha, A. D., Gelaw, B. K., et al. Drug utilization review of ciprofloxacin in the outpatient department of BoruMeda Hospital, South Wollo Zone, Amhara Region, Ethiopia. (2014) Int J Basic Clin Pharmacol 3(1): 171-178.
Pubmed| Crossref| Others
- 17. Muhammed, O.S. Drug use Evaluation of Ciprofloxacin in the Outpatient Department of Dessie Referral Hospital (DRH), North East Ethiopia. (2015) Austin Emerg Med 1(1): 1001.
Pubmed| Crossref| Others