Effect of Chromium(III) -Amino acid (1:3)Complexes on High Sucrose Induced Insulin Resistance, Lipid Abnormalities and Oxidative Stress in Male Sprague Dawley Rats
Nagabhushan Reddy Konapalli1, Anil. Sakamuri1, Kalashikam Rajender Rao1, Rajendher Reddy M2, Praveen Kumar Y2, Sirasani Satyanarayana2 and Raghunath Manchala1*
Affiliation
- 1National Institute of Nutrition, Indian Council of Medical Research, Hyderabad, 500 007, India.
- 2Department of Chemistry, Osmania University, Hyderabad, 500 007, India.
Corresponding Author
Dr. Raghunath Manchala, Scientist-F & Head, Division of Endocrinology & Metabolism, National Institute of Nutrition, Jamai Osmania, Hyderabad -500 007, Andhra Pradesh, India. Tel:91-40-27197235/ FAX: 91-40-27019074; E-mail: mraghunath55@yahoo.com
Citation
Manchala, R., et al. Effect of Chromium (III) -Amino Acid (1:3) Complexes on High Sucrose Induced Insulin Resistance, Lipid Abnormalities and Oxidative Stress in Male Sprague Dawley Rats. (2015) J Dia Obes 2 (1): 16- 23.
Copy rights
© 2015 Manchala, R. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Chromium (III) - Amino acid complexes; Sucrose; Diabetes; Insulin Resistance; Oxidative Stress & Anti oxidant enzymes.
Abstract
This study was carried out to assess the anti-hyperglycaemic effects if any of novel synthetic binary chromium (III)-amino acid complexes in a high sucrose (HS) induced insulin resistance (IR) and or impaired glucose tolerant male Sprague Dawley (SD) rat model. Aqueous solutions of chromium chloride hexahydrate (CrCl3.6H2O) and D/L amino acids were mixed in 1:3 molar ratios, refluxed at 80°C for 4 hours and the resultant crude greenish-violet solid was extracted with acetone, dried in a hot air oven and their elemental composition and structure were determined. The chromium- amino acid [Cr-(AA)3] complexes were administered orally (~45 μg/kg body weight/day up to 12 weeks) to adult male SD rats in which IR and or impaired glucose tolerance were induced by feeding a HS diet. In line with literature reports, Cr-D-(Phe)3 complex improved insulin sensitivity and oral glucose tolerance in the HS fed rats. Interestingly, Cr-L-(Phe)3 complex also improved these parameters. Increased phosphorylation of insulin receptor substrate- 1 and Akt and increased glucose transporter-4 expression in skeletal muscle were associated with these changes suggesting modulation of insulin sensitivity by Cr-L/D-(Phe)3 complexes. Further, sub chronic administration of Cr-(AA)3 complexes significantly improved lipid metabolism. Modulation of oxidative stress and/ or antioxidant status of the animals seem to be associated with/underlie these beneficial effects in HS induced insulin resistant SD rats.
Introduction
Type 2 diabetes (T2D) is an emerging health problem worldwide, with the number of cases projected to double to 350 million by the year 2025[1]. A major cause of the increased prevalence is the growing epidemic of obesity[2]. Chronic over nutrition, sedentary lifestyle and the lack of physical activity have contributed to the increasing prevalence of diabetes[3].
Insulin resistance (IR), an impaired responsiveness of the body to insulin, is a prediabetic stage in the transition from obesity to full-blown T2D[4]. IR is observed long before the development of diabetes and early identification and treatment of persons in prediabetic state can delay their progression to fullblown diabetes and associated cardiovascular diseases etc[5]. Therefore, IR is an attractive target for therapeutic intervention ahead of the development of diabetes. The currently accepted therapies include pharmacological treatment, calorie restriction and physical exercise, which are effective, but none is deemed the ultimate cure. Although the currently popular medications such as thiazolidine diones and biguanides can improve insulin sensitivity, they have serious adverse effects. Therefore, new agents need to be developed that augment insulin sensitivity and help overcome the complications associated with IR and T2D.
In this context, the design and characterization of effective and safe nutritional supplements that can alleviate IR represents an attractive strategy. Among them the micronutrient, chromium (Cr) has been gaining popularity as a dietary supplement to improve the actions of insulin under insulin-resistant conditions. The potential role of Cr in regulating blood sugar was first indicated in the late 1950s by Mertz and Schwarz[6]. Acting as a cofactor for insulin, Cr enhances glucose utilization by target tissues[7]. It facilitates insulin binding to its receptors, activates insulin receptor kinases and inhibits insulin receptor phosphatases[8]. Dietary deficiency of Cr is positively associated with the risk of diabetes and its complications[9] and dietary Cr supplementation lowers blood glucose concentrations and improve lipid profile in diabetic patients[10,11]. In a clinical trial, Martin[12] demonstrated improved insulin sensitivity in T2D subjects treated with Cr. In contrast, some reports claim Cr to be not an essential trace element for mammals[13] and that Cr treatment may not benefit diabetic subjects[14,15]. Therefore further studies are necessary to understand the potential role of Cr in treating IR and T2D[16]. Based on the better bioavailability of Cr reported from low molecular-weight (LMW) organic Cr complexes and the identification that the biologically active form of Cr is a complex with an oligopeptide complex, several LMW organic Cr complexes have been designed and evaluated as potential therapeutic agents to counter the diminished effect of insulin in T2D[17]. In view of the foregone literature the present study aimed to investigate the impact of sub chronic treatment with binary complexes of Cr with some amino acids reported to be present in GTF of yeast , viz Gly, Cys and Lys on high sucrose (HS) induced IR and / or impaired glucose tolerance in male SD Rats. Considering the reported beneficial effects of Cr-D-(Phe)3 complex, we assessed the effects of the binary complexes of L- and D-Phe with Cr in this model. In addition, it assessed the effects of Cr-(AA)3 on HS induced lipid abnormalities and oxidative stress (OS) in this rat model.
Materials and Methods
The micro protein assay kit was purchased from Thermo Scientific (USA) and antibodies to GLUT-4, pAkt, Akt, IRS-1 and PI3-K were from Calbiochem and Merck Millipore Company, UK. Unless stated otherwise, all other chemicals & reagents used in this study were of analytical grade obtained from Sigma Chemicals Co.
Synthesis of Cr-(AA)3 complexes
Cr-(AA)3 complexes were synthesized by mixing aqueous solutions of 10mM CrCl3.6H2O (50mL) and 30mM amino acid (50mL) and heating at 80°C and refluxing for 4 hours. The homogeneous green reaction mixture was freeze-dried and the greenish-violet solid obtained was washed with acetone and dried in a hot air oven. The synthetic protocol used was closer to that reported by Abdel-Monem et al[18]. The effects of these Cr-(AA)3 complexes were evaluated in HS induced IR impaired glucose tolerance in male SD rats.
Animals
The animal experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) approval (IAEC No: P42/12-2011/MR) at the National Centre for Laboratory Animal Sciences (NCLAS), National Institute of Nutrition (NIN), Hyderabad and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) (Regd. No. 154/1999/CPCSEA) and the experiment was carried out as per the animal ethical norms.
Two month old male SD rats (n = 48) were obtained from NCLAS, NIN and housed individually in polypropylene cages with wire mesh bottom and maintained at 220C ± 2, under standard lighting conditions (12- hour light or dark cycle). They had free access to water and food (control and high sucrose diet), whose composition is given in table 1.
Table 1: Composition of the diets used in the experiments
| Ingredients | Control diet (g/kg) | Sucrose diet (g/kg) |
|---|---|---|
| Casein | 250 | 250 |
| Starch | 545 | 0 |
| Oil | 100 | 100 |
| Cellulose | 50 | 50 |
| Sucrose | 0 | 545 |
| L-cysteine | 3 | 3 |
| Choline chloride | 2 | 2 |
| Mineral mix¹ | 40 | 40 |
| Vitamin mix² | 10 | 10 |
¹mineral mix composition (g/kg): calcium phosphate, dibasic, 500; magnesium oxide, 24; Potassium citrate, 220; potassium sulphate, 52; sodium chloride, 74; magnesium sulfate, 0.55; cupric carbonate, 0.3; Potassium iodide, 0.01 ; ferric citrate, 6; manganous carbonate, 3.5; sodium selenite, 0.01; zinc carbonate, 1.6. ²vitamin mix composition (g/kg): vitamin A(synthetic vitamin A blended in corn oil), 0.8; cholecalciferol, 1.0; vitamin E acetate (d,l- alpha –tocopherol acetate), 10.0; menadione sodium bisulphate, 0.08; biotin 1%, 2.0; cyanocobalamin 0.1%, 1.0; folic acid, 0.2; nicotinic acid, 3; calcium pantothenate, 1.6; pyridoxine-HCL, 0.7; riboflavin,0.6;thiamine-HCL, 0.6.
Control and high sucrose diets
Synthetic diets were prepared as per American Institute of Nutrition (AIN)- 93G recommendations. Control diet for the rats contained starch- (carbohydrate) 54.5%, casein- 25%, oil- 10%, cellulose- 5%, mineral mixture- 4%, vitamin mixture- 1%, L-cysteine- 0.3%, and choline chloride- 0.2%. The HS diet contained 54.5% sucrose instead of starch while the composition of the other ingredients remained the same (Table 1).
Experimental design
Rats were fed a rodent chow diet ad libitum, for one week initially. They were then randomly divided into six groups consisting 8 animals in each group. Group- C: fed control diet and DW. Group- S: fed HS diet and DW. Group- S1: fed HS diet and DW containing Cr-D-(Phe)3. Group-S2: fed HS diet and DW containing Cr-L-(Phe)3. Group-S3: fed HS diet and DW containing Cr-(Gly)3. Group-S4: fed HS diet and DW containing Cr-L-(Cys)3. Based on the calculated water intake of the rats, Cr-(AA)3 complexes were administered through drinking water to provide a dose of ~45 μg/ kg body weight/ day. The daily dosage of Cr (III) to the rats was based on an earlier report on the beneficial effects of Cr (III) in a rat model[19]. The drinking water containing the Cr-(AA)3 was freshly prepared every day. These animals were maintained on their respective diets for a period of 12 weeks from the day of HS feeding and Cr-(AA)3 complex supplementation.
Fasting glucose, Insulin, oral glucose tolerance and insulin resistance
Body weights of the animals were measured at the beginning and end of experimental period. The experimental animals and their controls were subjected to the oral glucose tolerance test (OGTT) after an overnight / 12-h fasting and collection of a blood sample from retro orbital sinus. Without delay, a glucose solution (40% in DW) was administered through a gastric gavage at a dose of 2.5g/kg body weight. Three more blood samples were collected at 30, 60,120 minutes after glucose administration. All blood samples were collected in 2 ml centrifuge tubes containing 2% sodium fluoride (100 μl/ml of blood) and kept on ice till centrifugation. From the whole blood, plasma was separated and stored at -200C until further use. Blood glucose concentrations were measured using a glucometer (Rite Check Blood Glucose Monitoring System, OK Biotech Co, Ltd., Taiwan) and plasma insulin concentration was measured using the Radio Immuno Assay (RIA) kit purchased from BRIT Mumbai, India. The area under the curve (AUC) of glucose and insulin during OGTT were computed by the trapezoidal method[20]. The indices of IR such as Homeostasis Model Assessment of IR (HOMA-IR) index and the ratio of glucose AUC to insulin AUC during OGTT were computed as described by us earlier[21].
Biochemical measurements
Plasma triglycerides[22,23], total cholesterol[24,25], and HDL- cholesterol[26,27] levels were measured using commercially available enzyme based assay kits (Biosystems, Barcelona, Spain).
Sample collection
At the end of the experimental period the animals were fasted overnight and sacrificed by cervical decapitation. Liver and skeletal muscle (Gastrocnemius) were excised quickly and frozen immediately in liquid nitrogen, and stored at -80°C until used.
Oxidative stress and antioxidant defence markers
Liver was weighed, minced and homogenized (10% w/v) in 50mM phosphate buffer (pH 7). The homogenate was centrifuged at 1000xg for 20min at 4°C and a portion of supernatant was used for the estimation of lipid peroxidation [Thiobarbituric acid reactive substances (TBARS)] & protein carbonyls using standard methods[28,29]. The remaining supernatant was further centrifuged at 12,000x g for 20min at 4°C to obtain the post-mitochondrial supernatant, which was used for the estimation of reduced glutathione[30] and to determine the activities of antioxidant enzymes: Catalase[31], Superoxide dismutase (SOD)[32] and Glutathione peroxidase (GPx)[33].
SDS- PAGE and Western blotting
Skeletal muscle was homogenised in Radio immune precipitation Assay (RIPA) buffer (150mM NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS and 50mM Tris, pH 7.2) containing 1μM phenyl methanesulfonylfluoride (PMSF) and 1:100 dilutions of protease inhibitor cocktail. The homogenate was centrifuged at 15,000 x g for 15 min at 4°C, the supernatant was collected and its protein concentration determined by bicinchoninic acid (BCA) method. Equivalent amounts of proteins were boiled in Laemmli sample buffer, resolved on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Membranes were incubated with appropriately diluted primary antibody for IRS-1 (1:2000), GLUT-4 (1:1000), Akt (1:1000) and pAkt (1:500) in blocking buffer followed by incubation with horseradish peroxidase coupled secondary antibodies. Immuno reactive bands were visualized using chemiluminescense reagents (Bio-Rad, CA, USA). The band intensity was measured with a scanning densitometer coupled with Bio-Rad PC analysis software.
Statistical analysis
All the values are expressed as Arithmetic Mean ± SEM, except HOMA IR which is given as Geometric mean ± SEM. The differences among different groups in various parameters was analysed by one-way analysis of variance (ANOVA) using SPSS statistics package (version 17.0) followed by posthoc least significant difference test (LSD). A probability value of p < 0.05 was considered to indicate the significance of the ratio and significant difference between means of different groups. All the results are reported as mean ± SEM.
Results
Characterization of Cr-(AA)3 complexes
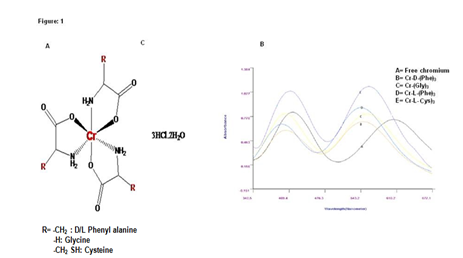
Purified Cr-(AA)3 complexes were characterized by using following methods. UV-Visible spectra were recorded with an Elico Bio spectrophotometer, model BL198 (Figure 1 and table 2). IR spectra were recorded on KBr disks on a Perkin- Elmer FT-IR-1605 spectrometer (Table 3). Elemental micro analysis (C, H and N) were carried out with a Perkin-Elmer 240 elemental analyser. ESI-MS mass spectra were recorded on ESIMS Micro mass Quattro Lc triple quadruple mass spectrometer with Mass Lynx software (Manchester, UK) in m/z.
Table 2: Uv-Vis λmax values of Cr-(AA)3 complexes.
| Cr(Aa) complexes | λ max(A) | λ max(B) | Δλ(A) | Δλ(B) |
|---|---|---|---|---|
| Free chromium | 417 | 602 | -- | -- |
| Cr-D-(Phe)3 | 400 | 547 | 17 | 55 |
| Cr-L-(Phe)3 | 398 | 545 | 19 | 57 |
| Cr-(Gly)3 | 402 | 549 | 15 | 53 |
| Cr-L-(Cys)3 | 412 | 558 | 05 | 44 |
Difference in λ-max indicates that complexation has taken place.
Figure 1: A: Proposed chemical structure Cr-(AA)3 complexes, B: Uv-Vis Spectra of Different Cr-(AA)3 complexes
Table 3: FTIR spectra of Cr(AA)3 complexes (strong peaks) in cm-1
| Cr. AA Complexes | N-H (3400- 3250) | C-H (3100- 2700) | C-O (1760- 1600) | C-O (1350- 1000) | C-N (1280- 1050) | Cr-O (540-450) | Cr-N (500-420) | S-H (1670- 1500) |
|---|---|---|---|---|---|---|---|---|
| Free-(L)Phe alanine | 3400 | 2875 | 1610 | 1315 | 1225 | -- | -- | -- |
| Cr-(L)Phenylalanine | 3407 | 2883 | 1615 | 1319 | 1261 | 458 | 453 | -- |
| Free-(D)Phenylalanine | 3405 | 2875 | 1610 | 1317 | 1228 | -- | -- | -- |
| Cr-(D)Phenylalanine | 3410 | 2886 | 1620 | 1325 | 1267 | 460 | 455 | -- |
| Free Cysteine | 3380 | 2868 | 1625 | 1328 | 1250 | -- | -- | 1584 |
| Cr-(L)Cysteine | 3385 | 2871 | 1628 | 1336 | 1256 | 462 | 452 | 1588 |
In FTIR, strong peaks for Cr-O, Cr-N indicate that complex formation has taken place.
Cr-D-(Phe)3 complex: Elemental analysis found: C, 47.84; H, 5.60; N, 5.92. The stoichiometry Cr-D-(Phe)3 3HCl 2H2O requires C, 52.00; H, 5.40; N, 7.29. The ESMS (expand) of the complex in methanolic solution registered signals at 546.1 and 165.8 representing, the tris chelate and the deprotonated ligand respectively. Formation of the complex was associated with C-O (2883 cm-1) and N–H (3407 cm-1) shifts in the IR-spectrum by about 17 and 20 cm-1 respectively. The broadening of the moderately sharp absorption band in the free ligand (2900–3100 cm-1) to about 600 cm-1 may be attributed to the reorganization in intramolecular hydrogen bonding after chelation. New absorption bands in the far IR region around 458 and 453 cm-1 can be assigned to the Cr–O and Cr–N bonds. The UV– V is spectrum of the methanolic solution of the complex registered bands at (400 nm) 15,670 cm-1 (m1) and (412 nm) 22,073 cm-1 (m2). The complex being greenish-violet in colour, the above two bands are due to the absorption in yellow and blue parts of the spectrum. These absorptions are due to the spin allowed transitions 4T2g ‹ 4A2g (m1) and 4T1g (F) ‹ 4A2g (m2). The third band m3 overlaps with UV absorption of the ligand. These observations suggest a hexa-coordinate environment around chromium (III). The pH of the aqueous solution of the complex is 4.5 and the presence of chloride demonstrates the presence of HCl in the lattice. Based on the stoichiometry, elemental analysis and spectral studies, the product obtained is a complex containing a 1:3 ratio of chromium to D- phenylalanine.
Cr-L-(Phe)3 complex: Elemental analysis found: C, 48.54; H, 5.55; N, 5.82. The stoichiometry Cr-L-(phe)3 3HCl 2H2O requires C, 50.00; H, 5.45; N, 7.18. The ESMS of the complex in methanolic solution registers signals at 545.3 and 164.8 representing, respectively, the tris chelate and the deprotonated ligand. Formation of the complex was associated with Formation of the complex was associated with m C‚O (2885 cm-1) and mN–H (3412 cm-1) shifts in the IR-spectrum by about 18 and 21cm-1 respectively. The broadening of the moderately sharp absorption band in the free ligand (2900–3100 cm-1) to about 600cm-1 may be attributed to the reorganization in intramolecular hydrogen bonding after chelation. New absorption bands in the far IR region around 428 and 423cm-1 can be assigned to the Cr–O and Cr–N bonds. The UV–V is spectrum of the methanolic solution of the complex registered bands at (398nm) 15,665 cm-1 (m1) and (545nm) 22,058 cm-1 (m2). The complex being greenish- violet in colour, the above two bands are due to the absorption in yellow and blue parts of the spectrum.
Cr-(Gly)3 complex: Elemental analysis found: C, 26.28; H, 4.41; N, 15.33 The stoichiometry Cr-(Gly)3 3HCl 2H2O requires C, 25.29; H, 4.40; N, 15.25. The ESMS of the complex in methanolic solution registers signals at 274.01 representing, respectively, the tris chelate and the deprotonated ligand. Formation of the complex was associated with Formation of the complex was associated with m C‚O (2880 cm-1) and mN–H (3409 cm-1) shifts in the IR-spectrum by about 16 and 20 cm-1, respectively. The broadening of the moderately sharp absorption band in the free ligand (2900– 3100 cm-1) to about 600 cm-1 may be attributed to the reorganization in intramolecular hydrogen bonding after chelation. New absorption bands in the far IR region around 450 and 455 cm-1 can be assigned to the Cr–O and Cr–N bonds. The UV–V is spectrum of the methanolic solution of the complex registered bands at (402 nm) 15,670 cm-1 (m1) and (549 nm) 22,073 cm-1 (m2). The complex being greenish-violet in colour, the above two bands are due to the absorption in yellow and blue parts of the spectrum.
Cr-L-(Cys)3 complex: Elemental analysis found: C, 47.84; H, 5.60; N, 5.92. The stoichiometry Cr-(L-Cys)3 3HCl 2H2O requires C, 52.00; H, 5.40; N, 7.29. The ESMS of the complex in methanolic solution registers signals at 546.1 and 165.8 representing, respectively, the tris chelate and the deprotonated ligand. Formation of the complex was associated with Formation of the complex was associated with m C‚O (2883 cm-1) and mN–H (3407 cm-1) shifts in the IR-spectrum by about 17 and 20cm-1, respectively. The broadening of the moderately sharp absorption band in the free ligand (2900– 3100 cm-1) to about 600 cm-1 may be attributed to the reorganization in intramolecular hydrogen bonding after chelation. New absorption bands in the far IR region around 450 and 455 cm-1 can be assigned to the Cr–O and Cr–N bonds. The UV–V is spectrum of the methanolic solution of the complex registered bands at (405 nm) 15,675 cm-1 (m1) and (420 nm) 22 079 cm-1 (m²). The complex being greenish- violet in colour, the above two bands are due to the absorption in yellow and blue parts of the spectrum.
Food intake and body weight
Food intake was comparable among all the groups studied (Table 4). Nevertheless, the body weights of rats fed HS diet (Group-S) were higher than those fed control diet (Group-C). Interestingly administration of Cr-D-(Phe)3 and Cr-L-(Phe)3 complexes but not the Cr-(Gly)3 and Cr-L-(Cys)3 complexes mitigated the changes in the bodyweights although they did not affect their food intake (Table 4).
Table 4: Mean counts (CFU/note) of microbial pathogens isolated from paper currency[53]
| Parameter | C | S | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|---|
| Body weight (gms) | 302 ± 5.76a | 337 ± 8.25b | 305 ± 9.28a | 302 ± 4.17a | 338 ± 7.53b | 322 ± 6.35a |
| Food intake (g/day) | 20.15 ± 1.47 | 18.97 ± 1.22 | 21.04 ± 0.47 | 19.58 ± 0.97 | 20.18 ± 1.31 | 20.37 ± 2.15 |
| Fasting plasma glucose (mmol/L) | 4.33 ± 0.16a | 6.11 ± 0.142b | 4.51 ± 0.175a | 4.57 ± 0.214a | 5.67 ± 0.358b | 5.97 ± 0.435b |
| Fasting plasma insulin(mU/ml) | 66.16 ± 9.2a | 140.3 ± 14b | 76.52 ± 7.5a | 78.53 ± 6.1a | 128.8 ± 9.1b | 138.5 ± 7.1b |
| Insulin sensitivity index(HOMA-IR) | 12.18 ± 0.53a | 37.42 ± 3.8b | 15.11 ± 0.8a | 14.89 ± 0.3a | 31.82 ± 2.08b | 34.08 ± 3.1b |
HOMA-IR: Homeostasis model of assessment for insulin resistance was calculated as fasting plasma insulin (μU/ml) x fasting plasma glucose (mmol/l)/22.5. Values given are mean ± S.E.M. except for HOMA IR which is given as Geometric mean ± S.E.M (n = 8), values in a row bearing different superscripts are different (p < 0.05) from one another by one way ANOVA followed by post hoc least significant difference (LSD) test.
Fasting plasma glucose, insulin and HOMA-IR
As expected, feeding HS diet (S) increased fasting glucose and insulin levels in the SD rats compared to controls (Group-C). As a consequence, the HOMA-IR was significantly higher in group-S than group-C rats. Chronic oral administration of Cr-D-(Phe)3 and Cr-L-(Phe)3 complexes, but not Cr-(Gly)3 and Cr-L-(Cys)3 complexes mitigated the HS induced changes in fasting glucose, insulin and HOMA-IR (Table 4).
Glucose tolerance and insulin sensitivity
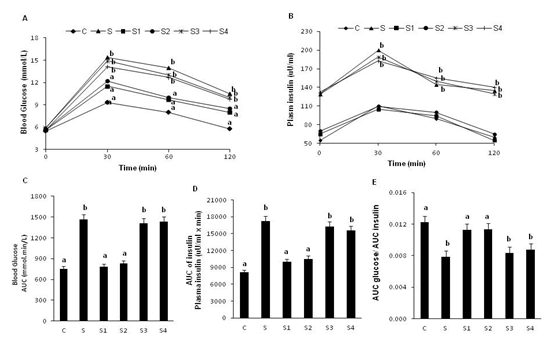
In line with the observations on fasting glucose, insulin and HOMA-IR mentioned above, rats fed HS diet had significantly higher (than group-C rats) values of AUC glucose and AUC insulin during the OGTT indicating that they developed insulin resistance and impaired glucose tolerance. Further, the ratio of AUC glucose / AUC insulin was significantly lower in group-S than group-C rats confirming the increase in post prandial insulin resistance. Interestingly, administration of Cr-D-(Phe)3 and Cr-L-(Phe)3 complexes to HS fed rats reversed the changes in the above parameters to that of control rats. However, administration of Cr-(Gly)3 and Cr-L-(Cys)3 complexes had no effect on these parameters (Figure 2).
Figure 2: Effect of oral supplementation of Cr-(AA)3 complexes on oral glucose tolerance and insulin levels. Panel A: Blood glucose levels, panel B: plasma insulin levels, panel C: AUC glucose, panel D: AUC insulin, panel E: AUC glucose/ AUC insulin. Values are mean ± S.E.M. (n = 8), values / bars without a common superscript ('a and b') different at P < 0.05 by one-way ANOVA followed by post hoc least significant difference (LSD) test.
Plasma lipid profile
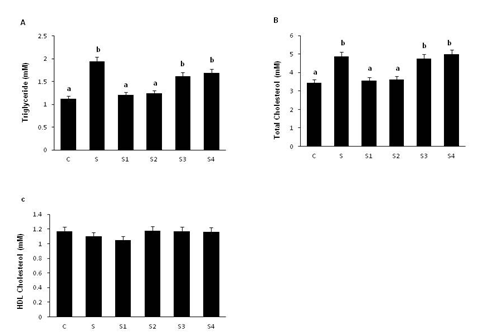
Plasma triglycerides and total cholesterol levels were significantly increased in the group-S rats compared to group C. Interestingly, plasma triglyceride and total cholesterol levels were decreased significantly in rats given Cr-D-(Phe)3 (group-S1) and Cr-L-(Phe)3 (group-S2) complexes but not in those given Cr-(Gly)3 (group-S3) or Cr-L-(Cys)3 (group-S4) complexes. HDL-cholesterol levels were comparable among the groups (Figure 3).
Figure 3: Effect of oral supplementation of Cr-(AA)3 complexes on serum lipid profile. Panel A: triglycerides, panel B: total cholesterol, panel C: HDL- cholesterol. Values are mean ± SEM (n = 8). Bars without a common superscript ('a and b') different at P < 0.05 by one-way ANOVA followed by post hoc least significant difference (LSD) test.
Hepatic oxidative stress markers and antioxidants
Malondialdehyde (MDA) and protein carbonyl levels were significantly increased in group-S compared to group-C. Interestingly (MDA) and protein carbonyl levels were significantly reduced in group's S1 & S2, but not in group's S3 & S4. The levels of reduced glutathione (GSH) were significantly lower in group-S compared to group-C. However the levels of GSH were significantly higher in group's S1 & S2, but not in groups S3 & S4. The levels of antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione -S- transferase (GST) and catalase (CAT) were significantly decreased in group-S compared to group-C. Interestingly, the changes in the activities of the antioxidant enzymes induced by feeding HS diet were significantly increased in group's S1 & S2, but not in groups S3 & S4 (Table 5)..
Table 5: Effect of Cr-AA complexes on hepatic oxidative stress markers and antioxidants.
| Parameter | C | S | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|---|
| MDA (nmol /mg protein) | 0.571± 0.025a | 1.42 ± 0.043b | 0.346± 0.043a | 0.392± 0.062a | 1.24 ± 0.025b | 1.37 ± 0.034b |
| protein carbonyls (umol/mg protein) | 1.79 ± 0.21a | 2.61 ± 0.21b | 1.42 ±0.16a | 1.38 ± 0.17 a | 2.63 ± 0.31b | 2.54 ± 0.35b |
| Reduced glutathione (GSH) (μg/mg protein) | 4.92 ± 0.38a | 3.92 ± 0.19 b | 4.75 ± 0.18a | 4.87 ± 0.24a | 4.07 ± 0.14b | 3.97 ± 0.31b |
| superoxide dismutase (SOD) (UA/mg protein) | 49.14 ± 1.43a | 39.54 ± 0.84b | 49.61 ± 2.35a | 48.41 ± 1.32a | 40.17 ± 0.47b | 41.17 ±0.25b |
| glutathione peroxidase (UB/mg protein) | 7.56 ± 0.34 a | 6.02 ± 0.34b | 7.48 ± 0.85a | 7.68 ± 0.31a | 5.78 ± 0.17b | 6.12 ± 0.24 b |
| Glutathione reductase (UC/mg protein) | 41.35 ± 0.84 a | 28.42 ± 0.76b | 39.34 ± 0.93a | 41.57 ± 0.57 a | 42.61 ± 0.34b | 41.82 ±0.54b |
| Catalase (UD/mg protein) | 65.46 ± 1.28a | 52.04 ± 1.58b | 63.59 ± 2.65a | 67.72 ± 1.57a | 41.07 ± 0.98b | 49.07 ±0.98b |
| glutathione -s-transferase (UE/mg protein) | 738.58 ± 13.65a | 609.57 ± 17.45b | 732.54 ± 14.57a | 723.5 ± 17.56a | 643.17 ± 18.20b | 621.57 ± 17.37b |
A- Amount of enzyme which gave 50% inhibition of pyrogallol autooxidation / min; B-mg of GSH consumed / min; C-mmol of NADPH oxidized / min; D- mmol of H2O2 decomposed/min; Emmol of GSH-CDNB conjugate formed/min. Values are mean ± S.E.M. (n = 8), values in a row bearing different superscripts are different (p < 0.05) from one another by one way ANOVA followed by post hoc least significant difference (LSD) test.
Skeletal muscle insulin signaling
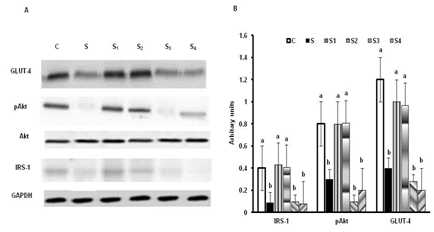
The expression of IRS-1, pAkt and GLUT- 4 were significantly lower in group-S compared to group-C. Interestingly these changes were mitigated in group's S1 & S2, but not in group's S3 & S4 rats. The amount of IRS1, phosphorylated Akt (pAkt) and GLUT-4 (translocated to membrane) were significantly higher in skeletal muscles of group's S1 & S2, but not in group's S3 & S4 (Figure 4).
Figure 4: Effect of oral supplementation of Cr-(AA)3 complexes on insulin signaling molecules. Panel A: Blots of GLUT4, pAkt, Akt, IRS1 and GAPDH, panel B: ratios of gel optical densities of protein bands. Values are mean ± S.E.M. (n = 8).Bars without a common superscript ('a and b') different at P < 0.05.
Discussion
Several studies have shown that elemental chromium (III) may play an important role in carbohydrate and lipid metabolism[34]. The biologically active form of Cr was identified as an oligopeptide chromodulin, a LMW chromium binding substance[35]. Based on these findings, LMW chromium complexes have been synthesized and evaluated as insulin-potentiating agents. Among the chromium-complexes used, chromium picolinate has gained popularity as a nutritional supplement. However, there have been some concerns regarding the mutagenic potential of chromium picolinate, which has been attributed to the OScaused by the picolinate ligand[36].
Cr-(AA)3 complexes used in this study were designed to mimic the activity of chromodulin, which is an oligopeptide complex of chromium with different amino acids. Compared with the picolinate ligand, the amino acid ligands used in the current study have better solubility at physiological pH and may also inhibit OS[37]. Earlier, a few studies have shown that the subchronic supplementation of Cr-D-(phe)3 have beneficial effects in T2D[19]. Since L- amino acids are biologically active forms which are metabolised in most of the organisms including animals, whereas D-amino acids are present in only few bacteria and are not metabolised by higher organisms including animals, in this study we investigated the efficacy of Cr-(AA)3 complexes derived from L-amino acids rather than D-amino acids. The Cr-(AA)3 complexes synthesized and utilized in this study are in line with that of GTF reported from yeast, with glucose uptake promoting properties and reported to contain amino acids. Also some earlier studies have reported that simple binary Cr-AA complexes may be sufficient to exert beneficial effects on glucose uptake by yeast in cultures[38].
High sucrose/fructose- induced IR animal model has been recommended for assessing the therapeutic efficacy of insulin sensitizers and drugs that are likely to affect insulin sensitivity. Therefore we selected HS induced IR model to study the efficacy of synthetic Cr-(AA)3 complexes in preventing / modulating HS induced IR in male SD rats. Results of the present study showed that HS feeding for 12 weeks resulted in fasting hyperglycemia, hypertriglyceridemia, hyperinsulinemia, glucose intolerance and IR in male SD rats. Further this was associated with impaired antioxidant potential and increased OS. These findings are consistent with those reported earlier[39,40]. The hypertriglyceridemia after HS feeding is known to result from the enhanced rate of hepatic VLDL- triglyceride synthesis[41] and/or a decrease in peripheral triglyceride clearance[42]. Further, increased delivery of triglycerides to the muscle impairs insulin action and interferes with the utilization of glucose, leading to hyperglycemia and hyperinsulinemia. That chronic oral supplementation of Cr-D/L-(Phe)3 complexes prevented the HS induced hyperglycemia and hyperinsulinemia in group's S1 and S2. SD rats appears to suggest that these positive effects could be attributed to the observed decrease in plasma triglyceride levels in these animals perhaps due to decreased synthesis and / or peripheral clearance.
The ability of insulin to stimulate glucose disposal under fasting condition was markedly impaired in rats fed HS diets, as evidenced by increased HOMA-IR which could be due to a decline in insulin sensitivity in peripheral tissues. That chronic oral supplementation of Cr-D/L-(Phe)3 complexes in HS fed rats prevented the abnormal rise in HOMA-IR that too by decreasing the fasting insulin levels significantly , indicates the ability of Cr-D/L-(Phe)3 complexes to increase insulin sensitivity of the target tissues and promote the clearance of circulating glucose. It is possible that the observed decrease in fasting glucose could also be due to decreased gluconeogenesis, which normally is the chief contributor to the fasting blood glucose levels. That chronic supplementation of Cr-D/L-(Phe)3 complexes to HS fed rats also improved oral glucose tolerance (decreased AUC glucose during OGTT) significantly indeed by decreasing the AUC insulin during OGTT suggests that Cr-D/L-(Phe)3 complexes improved the insulin sensitivity of the target tissues significantly. That binary complex of Cr with L. Phe (but not with Gly or L-Cys) was as effective as that with D. Phe seems to suggest binary complexes of Cr with only a few (but not all) AAs may be effective in this regard.
Defects in insulin signalling cascade have been shown to underlie impaired glucose utilization and proposed to play a key role in the pathogenesis of IR[43]. It is known that tyrosine phosphorylation of IRS 1 in response to insulin stimulation, generally increases the association of pIRS-1 with membrane bound PI-3 kinase, stimulating PI-3 kinase activity (by dissociating the 110 KDa catalytic subunit from the 85 KDa regulatory subunit) , which in turn activates serine/threonine protein kinase-B (PK-B or Akt). The active Akt ultimately leads to an enhancement of insulin stimulated glucose uptake / disposal by translocating intracellular Glut 4 to plasma membrane[44]. Our results reveal that the chronic feeding of HS diet significantly decreased the the levels of IRS 1 , pAkt (but not Akt per se) and total GLUT-4 levels, whereas chronic supplementation of Cr-D/L-(Phe)3 complexes (but not those with Gly or L-Cys) significantly increased the expression of IRS1 and Glut 4 and pAkt suggesting the correction of HS feeding induced impairment of insulin signalling in the skeletal muscle ,the insulin responsive target tissue important specially in post prandial clearance of circulating glucose. These findings also support the well-known concept that Cr may act by augmenting insulin signalling.
The development of OS, an imbalance between proand antioxidant status, has been shown to play an important role in mediating IR. Therefore, we studied whether increased OS plays a role in the HS induced IR and diabetes in this rat model and also its modulation by supplementation with Cr-AA peptides. Increased lipid peroxidation and protein carbonyl levels along with decreased hepatic GSH levels, and activities of antioxidant enzymes in HS fed rats, clearly indicate the development of OS and impaired anti-oxidant status in these animals. The increase in catabolism of sucrose and its product fructose could be associated with the cellular energy depletion that can increase the susceptibility of cells to lipid peroxidation[45]. Furthermore, it has been postulated that fructose can accelerate free radical production similar to glucose. Reactive oxygen species (ROS) can themselves reduce the activity of antioxidant enzymes[46]. The decreased SOD activity in HS fed rats may be due to enhanced protein glycation by fructose as it is a more reactive reducing sugar compared to others (glucose and lactose)[47]. The reduced GSH levels could be due to its increased utilization to trap free radicals, and/ or decreased regeneration as evident with the lower activity of glutathione reductase enzyme. That chronic supplementation of Cr-D/L-(Phe)3 complexes reversed the changes induced by HS in oxidative stress and anti-oxidant enzyme activities in addition to correcting the insulin resistance, impaired glucose tolerance and lipid metabolism / profile in these rats seems not only to suggest their modulation by Cr-AA complex but also that altered oxidative stress and ant oxidant activity could underlie the HS diet induced impairment of ininsulin sensitivity and glucose tolerance in these rats. As OS has been suggested to be one mechanisms for the detrimental effects of sucrose/fructose, the antioxidant potential of Cr-D/L-(Phe)3 complexes appears to be one of the mechanisms by which these complexes prevented IR and impaired glucose tolerance in HS fed rats. Some studies have indeed linked ROS production and OS to IR[48]. Several clinical trials, have also demonstrated that treatment with vitamin-E, vitamin-C, or glutathione improves insulin sensitivity in insulin resistant individuals and/or patients with T2D[49,50].
In summary, the present study shows that chronic administration of a new Cr-(AA)3 complex's Cr-D-(Phe)3 and Cr- L-(Phe)3 alleviate HS diet induced IR and impaired glucose tolerance probably due to the correction of changes in oxidative stress and anti-oxidant status and also mediated by augmenting insulin signalling. Cr-(AA)3 complexes treatment also attenuates hepatic triglyceride levels and lipid accumulation. These results suggest that nutritional supplementation with Cr-(AA)3 complexes may have potential therapeutic value in the better management of the IR associated with metabolic syndrome.
Conflict of Interest: The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding: This work was supported by a research grant (to M.R.) from the Department of Biotechnology (DBT), Government of India, New Delhi, India (project no. BT/PR-12615/FNS/20/409/2009).
Acknowledgment: The authors (N.R.Konapalli) acknowledge the Department of Science and Technology for awarding DSTINSPIRE research fellowship.
References
- 1. Zimmet, P., Alberti, K.G.M.M., Shaw, J. Global and societal implications of thediabetes epidemic. (2001) Nature 414: 782–787.
- 2. Popkin, B.M. Recent dynamics suggest selected countries catching up to US obesity. (2010) Am J ClinNutr 91(1): 284S–288S.
- 3. Koh-Banerjee, P., Wang, Y., Hu, F.B., et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. (2004) Am J Epidemiol 159(12): 1150–1159.
- 4. Muoio, D.M., Newgard, C.B. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. (2008) Nat Rev Mol Cell Biol 9(3): 193–205.
- 5. Gillies, C.L., Abrams, K.R., Lambert, P.C., et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetesin people with impaired glucose tolerance: systematic review and meta-analysis. (2007) BMJ 334: 299.
- 6. Mertz, W., Schwarz, K. Relationship of glucose tolerance to impaired intravenous glucose tolerance of rats on stock diets. (1959) Am J Physiol 196(3): 614–618.
- 7. Anderson, R.A. Chromium, glucose tolerance, and diabetes. (1992) Biological Trace Element Research 32(1-3): 19-24.
- 8. Vincent, J.B. The biochemistry of chromium. (2000) J Nutr 130(4): 715-718.
- 9. Jeejeebhoy, K.N., Chu, R.C., Marliss, E.B., et al. Chromium deficiency, glucose intolerance, and neuropathy reversed by chromium supplementation, in a patient receiving long-term total parenteral nutrition. (1977) Am J ClinNutr 30(4): 531–538.
- 10. Broadhurst, C.L., Domenico, P. Clinical studies on chromium picolinate supplementation in diabetes mellitus-a review. (2006) Diabetes TechnolTher 8(6): 677–687.
- 11. Vincent, J. B. "Beneficial Effects of Chromium(III) and Vanadium Supplements in Diabetes". (2012). Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome. pp. 381–391.
- 12. Di Bona, K.R., Love, S., Vincent, J.B., et al. Chromium Is Not an Essential Trace Element for Mammals: Effects of a "Low-chromium" Diet. (2011) J BiolInorgChem 16(3): 381-390.
- 13. Martin, J., Wang, Z.Q., Zhang, X.H., et al. Chromium picolinate supplementation attenuates body weight gain and increases insulin sensitivity in subjects with type 2 diabetes. (2006) Diabetes Care 29(8): 1826–1832.
- 14. Kleefstra, N., Houweling, S.T., Jansman, F.G., et al. Chromium treatment has no effect in patients with poorly controlled, insulin-treated type 2 diabetesin an obese Western population: a randomized, double-blind, placebocontrolled trial. (2006) Diabetes Care 29(3): 521–525.
- 15. Vincent, J. B., Love, S. T. The Need for Combined Inorganic, Biochemical, and Nutritional Studies of Chromium (III). (2012) ChemBiodivers 9(9): 1923–1941.
- 16. Gunton, J.E., Cheung, N.W., Hitchman. R., et al. Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance. (2005) Diabetes Care 28(3): 712–713.
- 17. Vincent, J.B. Recent advances in the nutritional biochemistry of trivalent chromium. (2004) ProcNutr Sec 63(1): 41-47.
- 18. Abdel-Monem, M.M., Mahamoud, M., Anderson, M.D. Novel chromium (III) alpha amino acid complexes. (2003) US patent 0228394.
- 19. Dong, F., Kandadi, M.R., Ren, J., et al. Chromium (D-phenylalanine)3 supplementation alters glucose disposal, insulin signaling, and glucose transporter- 4 membrane translocation in insulin-resistant mice. (2008) J Nutr 138(10): 1846–1851.
- 20. Mathews, J.N., Altman, D.G., Campbell, M.J.et al. Analysis of serial measurements in medical research. (1990) BMJ 300(6719): 230–235.
- 21. Padmavathi, I.J., Kishore, Y.D., Venu, L., et al. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. (2009) ExpPhysio l94(6): 761–769.
- 22. Padmavathi, I.J., Kishore, Y.D., Venu, L., et al. Prenatal and perinatal zinc restriction: effects on body composition, glucose tolerance and insulin response in rat offspring. (2009) ExpPhysio l94(6): 761–769.
- 23. Fossati, P., Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produce hydrogen peroxide. (1982) Clinchem 28(10): 2077-2080.
- 24. Allain, C.C., Poon, L.S., Chan, C.S.G., et al. Enzymatic determination of total serum cholesterol. (1974) ClinChem 20(4): 470-475.
- 25. Meiattini, F., Prencipe, L., Bardelli, F., et al. The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. (1978) ClinChem 24(12): 2161-2165.
- 26. Grove, T.H. Effect of reagent pH on determination of high-density lipoprotein cholesterol by precipitation with sodium phosphotungstate-magnesium. (1979) ClinChem 25(4): 560-564.
- 27. Burstein, M., Scholnick, H.R., Morfin, P. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. (1970) J Lipid Res 11(6): 583-595.
- 28. Balasubramanian, K.A., Manohar, M., Mathan, VI. An unidentified inhibitor of lipid peroxidation in intestinal mucosa. (1988) BiochimBiophysActa 962(1): 51–58.
- 29. Uchida, K., Kanematsu, M., Sakai, K., et al. Protein-bound acrolein: potential markers for oxidative stress. (1998) PNAS 95(9): 4882–4887.
- 30. Hissin, P.J., Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. (1976) AnalBiochem74(1): 214–226.
- 31. Aebi, H. Catalase in vitro. (1984) Methods Enzymol 105: 121–126.
- 32. Marklund, S., Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. (1974) Eur J Biochem 47(3): 469–474.
- 33. Paglia, D.E., Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. (1967) J Lab Clin Med 70(1): 158–169.
- 34. Dutta, K., Podolin, D.A., Davidson, M.B., et al.Cardiomyocyte dysfunction in sucrose-fed rats is associated with insulin resistance. (2001) Diabetes 50(5): 1186–1192.
- 35. Vincent, J.B. Quest for the molecular mechanism of chromium action and its relationship to diabetes. (2000) Nutr Rev 58(3 Pt 1): 67–72.
- 36. Stearns, D.M., Wise, J.P.S,r, Patierno, S.R., et al. Chromium (III) picolinate produces chromosome damage in Chinese hamster ovary cells. (1995) FASEB J 9(15): 1643–1648.
- 37. Nukuna, B.N., Goshe, M.B., Anderson, V.E. Sites of hydroxyl radical reaction with amino acids identified by (2)H NMR detection of induced (1)H/(2)H exchange. (2001) J Am ChemSoc 123(6): 1208–1214.
- 38. Karthikeyan, K.S., Nagababu, P., Sivarama,Sastry. K., et al. Metabolism and Glucose Tolerance Factor Activity of Synthetic Amino acid- Chromium Complexes in Yeast. (2010) Curr Trends Biotechnol Pharm 4: 908-916.
- 39. Dutta, K., Podolin, D.A., Davidson, M.B., et al. Cardiomyocyte Dysfunction in Sucrose-Fed Rats Is Associated With Insulin Resistance. (2001) Diabetes 50(5): 1186 –1192.
- 40. Reddy, S.S., Karuna, R., Bhaskar, R., et al.Prevention of insulin resistance by ingesting aqueous extract of Ocimum sanctum to fructose-fed rats. (2008) HorMetab Res 40(1): 44-49.
- 41. Zavaroni, I., Chen, Y.D., Reaven, G.M. Studies of the mechanism of the fructose induced hypertriglyceridemia in the rat. (1982) Metabolism 31(11): 1077-1083.
- 42. Mayes, P.A. Intermediary metabolism of fructose. (1993) Am J ClinNutr 58(5 Suppl): 754S-765S.
- 43. Shulman, G.I. Cellular mechanisms of insulin resistance. (2000) J Clin Invest 106(2): 171-176.
- 44. Carvalho, E., Rondinone, C., Smith, U. Insulin resistance in fat cells from obese zucker rats- evidence for an impaired activation and translocation of protein kinase B and glucose transporter 4. (2000) Mol cell Biochem 260(1-2): 7-16.
- 45. Rajasekar, P., Ravichandran, M.K., Anuradha, C.V.Intraperitonial L- carnitine regulates lipid metabolism and reduces oxidative stress in fructose- induced hyperlipidemic rats. (2005) Diabetol Croat 34(3): 87-94.
- 46. Datta, K., Sinha, S., Chattopadhay, P. Reactive oxygen species in health and diseases. (2000) Natl Med J India 13(6): 304-310.
- 47. McPherson, J.D, Shilton, B.H., Walton, D.J. Role of fructose in glycation and cross- linking of proteins. (1988) Biochemistry 27(6): 1901-1907.
- 48. Paolisso, G., Giugliano, D. Oxidative stress and insulin action. Is there a relationship? (1996) Diabetologia 39(3): 357-363.
- 49. Evans, J.L., Goldfine, I.D. Alpha-lipoic acid: a multi-functional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. (2000) Diabetes TechnolTher 2(3): 401-413.
- 50. Jacob, S., Lehman, R., Rett, K., et al. Oxidative stress and insulin action: a role for antioxidants. In: Packer L, Rosen P, tritschler HJ, King GL. (2000) Antioxidents in Diabetes Management. Marcel Dekker, New York, pp. 319-338