Effect of Solar Drying Method on the Proximate Composition, Microbial Load, and Mycotoxin Concentration of Plantain (Musa Paradisiaca) Flour
Afia Sakyiwaa Amponsah*, Kwame Duodu Ansah, Moses Kwaku Golly and Noah Kwasi Nyenah
Affiliation
School of Applied Sciences and Technology, Sunyani Technical University, P. O. Box 206, Sunyani, Bono Region, Ghana
Corresponding Author
Afia Sakyiwaa Amponsah, School of Applied Sciences and Technology, Sunyani Technical University, P. O. Box 206, Sunyani, Bono region, Ghana, Tel: +233(0)208267801; Email: verosakyi306@yahoo.com
Citation
Amponsah, S.A., et al. Effect of Solar Drying Method on the Proximate Composition, Microbial Load, and Mycotoxin Concentration of Plantain (Musa Paradisiaca) Flour. (2020) J Food Nutr Sci 7(1): 38-44.
Copy rights
© 2020 Amponsah, S.A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Aflatoxins; Molds; Yeast; Plantain; Moisture; Sun-drying
Abstract
Processing plantain into flour increases the shelf-life of the product; therefore, making drying along the value chain of the plantain fruit very important.The purpose of this study was to assess the impact oftwo drying methods on the quality of plantain flour. Two solar drying methods: indirect solar drying (ISD) and direct solar drying (DSD) methods were used. Plantain flour samples from the two drying methods were assayed for their proximate composition, microbial load, and mycotoxin (aflatoxins) concentration. The result of the laboratory analysis showed that DSD had a significantly higher incident of both yeast and mould count (p<0.05) in the two samples. The DSD again recorded a significantly higher level of moisture (p<0.05) compared to the indirectly dried sample. There was no significant (p>0.05) difference in the aflatoxin-bearing fungi identified in both the direct and the indirect dried plantain flour samples. Two strains of Aspergillus (flavus and niger) were isolated in this study. This study concludes that the indirect solar drying method is more appropriate to use in drying than the direct or open sun drying for quality product development.
Introduction
Plantain is a species of the genus Musa, and it is believed to have originated in Southeast Asia[1]. Africa, Asia (India), and tropical America are known to have particular types of plantain which are the horn and the French plant ains[1]. Plantain as a staple crop, has fed and nourished populations around the world for generations and are a rich source of complex carbohydrates, vitamins and minerals, and are easily digestible[2]. According to Amaya (2018)[3], Ghana is the second highest producer of plantain in the world with an annual production of 3.95 metric tons compared with 4.31 metric tons per annum by Cameroon. Other producers with significant quantities include; Uganda, Columbia, Nigeria, Philippines, Peru, Cote d’Ivoire, Myanmar and the Democratic Republic of the Congo. Plantains are highly perishable, regardless of their economic value. Thus, they are very costly during the lean period of shortage while they cost less during the period of abundance where most go waste. It is postulated that postharvest losses could reach 30 – 40%[4].
It is imperative that plantain produced in the sub-region can be used to address and reduce the incidence of food insecurity due to the high production levels every year and could also increase the income level of plantain farmers and marketers. This objective can be achieved mainly through the processing of the produce, which will aid in increasing their self-life, thus available for the future[5]. One such approach is converting the plantain into powder after drying. Food drying is one step in the post-harvest management chain. According to Sabarez (2016)[6] as early as 12000 BC, people from the Middle East and Oriental cultures actively dried foods in the hot sun.
Furthermore, in the late 1700s, a unit for drying fruits and vegetables at a controlled temperature was developed by the French. In recent years, especially in the developed world, sophisticated dryers are designed and used by industries to replace the natural sun drying methods. Drying is purposely done to increase the storability level of the produce at the same time, reducing undesired changes in appearance, texture, flavor and nutritional value[7]. Drying is also done to inhibit the growth of bacteria, yeasts and mould through the removal of water[8]. The presence of moisture encourages the growth of fungi during storage[9].
In the food industry fungi is a menace as it is known to be a significant agent in food deterioration or spoilage[9]. Fungi are ubiquitous[10] and are primarily saprophytic[11] utilizing non-living organic material for growth and development as a nutrient source. During their growth, besides primary metabolites, fungi produce secondary metabolites, called mycotoxins acquiring a competitive edge against other microorganisms[11]. Aflatoxin, a mycotoxin created by Aspergillus flavus and Aspergillus parasiticusis naturally occurring are very robust teratogenic, mutagenic and carcinogenic compounds[12]. Alat oxin are of significant concern to the food industry. However, food contamination with aflatoxin is associated with warm and dry environmental conditions and is of a significant health concern, mostly in hot, and humid countries[13,14]. Large doses of aflatoxins in diet can cause serious health complications and consequences which mostly affect theliver and kidneys, but can also affect the reproductive organs[15]. Hence, the need for assessing food produce for permissible tolerant levels for human and livestock is essential.
Plantain stores well in the form of flour but inferior drying methods could enhance the activities of mould or fungus which produce mycotoxins. The study seeks to identify the effect of direct and indirect solar drying methods on the quality of plantain flour. Explicitly, the average moisture content, proximate (chemical) composition, level of microbial infections and average aflatoxin concentrations were assessed after direct and indirect sun drying methods.
Materials and Methods
Sample source and preparation
The false horn plantain fruit (Musa paradisiaca) in the green matured stage locally known as “apantu” was purchased from the Nana Bosoma market in Sunyani, Ghana. Sunyani is located in the Bono Region of Ghana, which is among the top three regions of plantain production in Ghana[4,16]. All analysis was performed at the Food Science and Microbial Biotechnology laboratory at Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. Figure 1 below shows the graphical abstract for the sample preparation. Briefly, the fruits were sorted (sizes, maturity and devoid of bruises), washed (under running water), peeled (using stainless steel knife) and chopped into small pieces (0.5cm thickness). Two portions of 15 kg each were labeled indirect solar drying (ISD) and direct solar drying (DSD) corresponding to the drying methods. The samples were spread out evenly as much as possible on the racks to allow for proper airflow. The drying period was ten (10) days for both drying methods. Drying efficiency was monitored over the ten days drying period. Five (5) pieces of chopped plantain were randomly selected from each drying method and weighed before drying initiation (day 0) and repeated every two days (2, 4, 6, and 10) till the end of the drying period. The samples from the two drying methods were milled and sieved (passed through a 20µm-mesh sieve), weighed separately and labeled appropriately. The different flour samples were used for the proximate composition, microbial (yeast & moulds) and the mycotoxins assay.
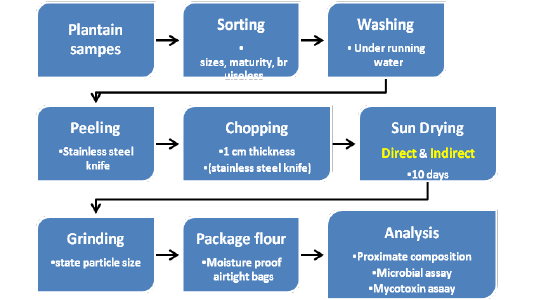
Figure 1: Graphical abstract of plantain sample preparation and sun drying
Drying methods
In this study, the indicated indirect solar drying (ISD) comprises drying in a solar cabinet drier. Samples of the chopped plantain were mounted on plastic mesh trays as one layer. The trays were set for drying inside the driers ‘ cabinet. The drying process uses solar heat to heat-up incoming air in the drier. Hot air absorbs moisture as it passes through the samples and discharges through the vents on the side of the dryer cabinet. On the other hand, the direct solar drying (DSD) system implies the drying of samples in open-air sunshine as practiced by the indigenous people.
Description of the solar drier
The solar dryer was designed as an enclosed rectangular chamber made of wood and raised by four wooden poles about 2 m from the ground level. The top surface of the dryer was tilted at an angle of 10 degrees and made of black plastic overlaid with a clear one. The components of the black plastic absorb the heat from the sun, which passes through the dryer interior. Inside the dryer, there were three trays made of wire mesh, on which the samples were placed to dry. The lower and upper sections of the dryer chambers featured vents that permitted air circulation and moisture removal, respectively, and the inner walls were painted black to maximize heat absorption. The temperature inside was increased to between 50 and 60°C with this system[17].
Proximate composition
The proximate (chemical) compositions of the pulverized sundried plantains were assayed employing standard methods (AOAC, 2002). The standard protocols were; moisture content (AOAC 934.01), crude protein (N x 5.30) (AOAC 988.05) employing a semi-automated Kjeldahl digester (UDK149 Automatic Kessler Nitrogen Meter, Shanghai), fat content was by Soxhlet extraction (AOAC 920.39), while the phenol-sulfuric acid method was carried out for the fibre content determination (AOAC 962.09) and ash (AOAC 945.05). The carbohydrate content of the plantain flour was determined by difference (Golly & Amadotor, 2013) as follows:
% Carbohydrate=100- %(moisture+ protein+fat+fibre+ash) Eq. 1
Microbial Assay
Sample preparation
Aseptic procedures were maintained throughout by ensuring all articles for the microbial examination were disinfected. Microbial samples were prepared by applying a modified technique[18]. Precisely 5g of each sample was weighed and blended in 45ml of peptone water solution. Serial dilutions were carried out to reduce a dense culture of cells to a more usable concentration. The serial dilutions were performed to 10-4. Figure 2 depcits the process flow for the microbial analysis.
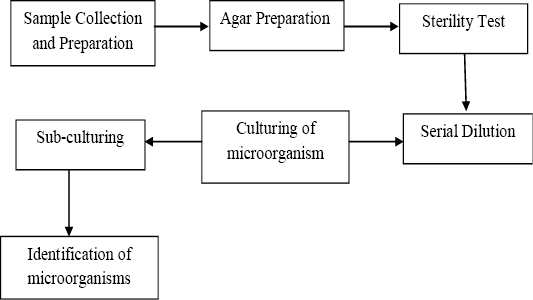
Figure 2: The process carried out in quantifying, isolating and identifying the microorganisms
Culture Media
The yeast and mould counts were carried out by the spread plate method on malt extract agar (MEA) (Oxiod Ltd. Basingstoke, England). The agar preparation was processed per the manufacturers’ specifications. Agar powder (25g) was blended in 500ml of distilled water and brought to the boil to dissolve completely. The agar slurry was sterilized by autoclaving at a temperature of 121°C, 0.1 Mpa pressure for 15 minutes and allowed to cool to 50 °C. The agar was mixed with 100 mg/L of chloramphenicol (Sigma-Aldrich Co., MO,USA) and 50 mg/L chlortetracycline hydrochloride (ICN Biochemicals Inc, Ohio, USA) to suppress the growth of bacteria.
Determination of Yeast and Mold count
A modified protocol of Golly et al. (2019)[19] was adopted. Briefly, 1.0 µL of aliquots were drawn from the fourth dilution onto the Petri dishes containing the sterilized MEA. The inoculated plates were evenly spread with a sterile bent rod and allowed to dry for 15 minutes at room temperature. The plates were inverted and incubated at 25 ºC for 120 h.
Culture examination and purification
The incubated plates were examined after 120 h for noticeable colonies which were counted and expressed as colony-forming units per gram (CFU/g). To obtain purified cultures, identifiable colonies were repeatedly streaked on fresh MEA and incubated appropriately until pure cultures were obtained and preserved appropriately.
Mycotoxin determination
The Reveal Q+ for Aflatoxin Quantitative Test coupled with Neogen’s line of AccuScan readers (USDA-GIPSA 2013-041) with modification was used to determine total aflatoxins in the plantain flour samples. The sample extraction and testing were accomplished as described below:
Sample Preparation and Extraction for Aspergillus Molds
A 65% of ethanol solution was prepared by mixing 6.5 parts of ethanol with 3.5 parts of doubled distilled water for the sample extraction. At least 75% of each milled flour sample passed through a 20µm-mesh sieve. The extraction slurry comprised 1 part of the sample to 3 parts 65% ethanol and vigorously shaken for 1 minute then vortex for 3 minutes to blend thoroughly. The extract slurry was stayed for 10 min then filtered through (0.45 um) microfilter membrane and used as the test sample.
Test procedure
A 10-fold dilution of the test sample was prepared by adding 100 µL of sample extract to 900 µL of 65% ethanol then vortex for 1 min. Appropriately labeled red and clear sample cups were placed into the sample cup racks accordingly. A 500 µL of sample diluents was added to each red sample dilution cup and mixed 100 µL of 10-fold diluted sample extract mixing thoroughly. An aliquot of 100 µL was then transferred into a new clear sample cup. A new Reveal Q+ for Aflatoxin test strip was placed into the sample cup, and a timer was set for 6 min, ensuring the test strips come into contact with the liquid. The strips were removed from the sample cups immediately after the 6 min and read with Neogen’s line of AccuScan readers within 1 min.
Statistical analysis
Data obtained from the yeast and mould count was subjected to unpaired T-test analysis using Graphpad Prism version 8.2.0. The proximate (chemical) composition result was entered into Microsoft Excel® 2016 to determine the Standard deviation for means comparison. Statistical difference in the samples was accepted at p<0.05 as significant.
Results and Discussion
Effect of drying method on sample appearance
The effects of the direct solar (sun) drying and indirect solar (sun) drying methods adopted in this study on the appearance of the slices plantain and resultant flours are depicted in Plate 1 below.
From Plate 1 (a & b), there was an observable difference in the appearance of the plantain slices after the ten (10) days drying period. The indirect solar-dried (Plate 1(a)) appeared relatively white compared with the direct solar-dried (Plate 1(b)) which appeared darker in colour. Both enzymatic and non-enzymatic browning action could be the reason for these observations. As cut surfaces are exposed to oxygen, colorless aromatic compounds become oxidized into colorful compounds impacting effect to dried food items. The closed environmental condition prevailing in the indirect solar drying approach reduced the intensity of this mechanism; hence, the superior colour in comparison with the direct solar-dried samples. The flour products from the two dried samples exhibited a similar trend with the indirect solar-dried plantain flour product appearing whiter (Plate 1 (c)) in comparison with the direct solar-dried plantain flour sample (Plate 1 (d)). Colour of food products is one of the critical sensory characteristics of importance to consumers in accepting or adopting a particular food product. Food processing techniques must result in visible esthetics properties of any food product for overall consumer acceptability. Indirect solar drying, which is relatively cheap, could be useful for farmers in low-income areas for maximum benefits.




Plate 1: Effects of drying method on plantain slices (a) direct solar-dried, (b) indirect solar dried and flour from dried plantain slices (c) indirect solar dried, and (d) direct solar-dried samples
Effect of drying method on sample weight
The effect of the drying methods and period on the mass (weight) of the plantain samples during the ten (10) days drying period were recorded (2 days intervals) and the results depicted in figure 3.
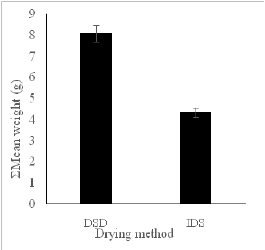
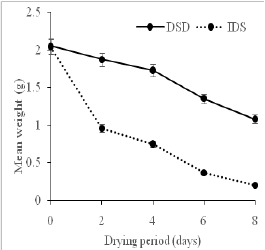
Figure 3: Effect of (a) drying methods on the total mean weight of the plantain sample. and (b) drying period on the mean mass of the plantain samples.
The total weighted mean for the plantain samples dried by direct (DSD) and indirect (ISD) approaches were 8.09g and 4.33g, respectively (Figure 3(a)). There was a significant difference (p<0.05) in the weight of samples dried with different methods. The dehydration processes were time-dependent such that as time (days of drying) increased more moisture was removed from the sample (Figure 3(b)). By inference, the indirectly dried sample had less moisture (water) content post drying weighing less relative to the direct sun method of drying, which weighed more than the indirectly dried sample. The mean weight difference of 3.76g (46.48%) represents the moisture content difference between the two samples. The experiment was conducted during the periods preceding the onset of the rainy season, which may have experienced some inconsistent weather condition[18]. This result could explain the effect of the open sun drying technique (DSD) by recording high mean weights which translate into high moisture content after the drying period indicating an inefficient drying process. The solar collector used in the ISD was able to trap and utilize the sun rays effectively for drying during the ten days. This outcome is expected to have an influence on the physico-chemical composition of the dried plantain flour. We, therefore, determined the proximate composition of the different flours per the drying methods and the outcome presented in the next section.
Proximate Composition
The proximate composition showed a high incidence of moisture content in the direct sun-dried sample as compared to the indirect sun-dried samples shown in Table 1 below. There was a significant difference (p > 0.05) in the moisture compositions of the two flour samples. Apart from crude fiber and crude ash which showed no significant difference (p > 0.05), there was a significant difference (p < 0.05) among the remaining proximate compositions. Furthermore, besides moisture and crude protein which significantly (p<0.05) reduced in composition after the two drying methods, the dried samples had significantly (p<0.05) increased crude fat, crude ash, crude fiber and carbohydrate compositions (Table 1).
Table 1: Composition of plantain flour from direct and indirect sun-drying methods
|
Index (%) |
Raw sample (control) |
DSD |
ISD |
|
Moisture |
59.59 ± 0.241a |
12.83 ± 0.447b |
4.38 ± 0.037c |
|
Crude protein |
7.68 ± 0.103a |
3.17 ± 0.101b |
2.41 ± 0.185c |
|
Fat |
1.49 ± 0.007b |
2.13 ± 0.004a |
2.18 ± 0.079a |
|
Crude ash |
1.45 ± 0.017b |
2.71 ± 0.012a |
3.07 ± 0.073a |
|
Crude fibre |
1.41 ± 0.037c |
3.63 ± 0.021b |
4.44 ± 0.002a |
|
Carbohydrate |
28.38 ± 0.567c |
79.16 ± 0.543b |
87.96 ± 0.300a |
|
Energy |
124.94* ± 0.589c (522.87) ** |
348.49* ± 0.756b (1,458.43)** |
381.10* ± 0.251a (1,595.28)** |
Values are mean ± stdv. (n=2). * values are in calories, and ** values are kilo Joules. Different superscripts along the row are significantly different (p<0.05). DSD, direct sun drying; IDS, indirect sun drying.
The differences in the proximate composition of raw plantain and processed plantain samples are as a result of the drying process. According to Stahl (2014)[6], processing enhances nutrient availability in dried food produce since there is a concentration of the nutrients as moisture (water) has being drawn out of the sample.
The proximate value for raw protein in table 1 is 0.27g, and that of direct and indirect sun-drying methods are 3.17 ± 0.101 and 2.41 ± 0.185 grams, respectively. Both direct and indirect drying sample recorded high protein content, but there was a difference in proximate composition value for the direct and indirect solar drying methods. The difference in proximate values occurred as a result of the difference in drying techniques. Adepoju, Sunday, and Folaranmi (2012)[19], reported that high-temperature results in protein denaturation and destruction. Therefore, the high accumulation of heat by the solar collector used for drying in the indirect drying method might have resulted in the difference in protein content between the two drying methods.
There was a significant increase in fat content for direct and indirect drying samples 2.13% and 2.18% compared to that of the raw plantain 0.20g (Table 1). Adepoju et al. (2012)[19], revealed that the increase in crude fat content might be due to the effect of the application of heat during processing. Dehydration leads to the concentration of other components of the dried material as moisture exits. There was no significant difference (p<0.05) in the fat composition of the dried samples. The fat content of the dried samples herein reported is lower than 3.9% for sun-dried plantain, as reported by Adepoju et al. (2012)[19]. This contrast could be as a result of variety difference in the samples and geographical variation with varied weather conditions.
There was an increase in crude fiber content for processed plantain flour samples for both direct and indirect drying method (Table 1). The fiber contents were 3.63% and 4.44% for direct sun-drying and indirect sun-drying correspondingly. This finding agrees with the results of Adepoju et al. (2012)[19], who reported a similar increase in fibre content of dried plantain over the raw sample. The increased fibre content after drying the plantain portrays a decrease in moisture content hence concentrating other components which is in agreement with Agoreyo, Akpiroroh, Orukpe, Osaweren, and Owabor (2011)[20]. The indirect sun-drying sample recorded the highest crude fibre content of 4.44% significantly different (p<0.05) from the direct sun-drying method. This observation is as a result of the lower moisture content in the indirect dried sample as depicted in Figure 3 above.
Comparing the moisture content of the two different drying methods used in the study, the indirect drying method was very effective and recorded a minimal amount of moisture content 4.38% compared to the direct drying method 12.83%. Both samples of the dried plantain had moisture content far less than the raw sample, which is a characteristic that makes plantain a high perishable fruit. The plantain fruit is associated with bumper harvest almost every year and consumed across the nation hence making preservation of the fruit very necessary. As the indirect drying method can reduce moisture content effectively, it positively impacts other areas by minimizing microbial growth, decay, and prolongs the shelf life of the plantain produce while reducing transportation cost as well. Carbohydrate content, on the other hand, has been reported to improve in a more digestible form when processes like drying are applied to agricultural produce. This observation may explain the increase in carbohydrate content for both samples, as shown in Table 1. The results support previous studies on sundried plantain[17].
Yeast & Mold Count
The yeast and mould count of the plantain flour from the two drying methods are presented in Figure 4. The flour from samples dried under direct solar (sun) conditions recorded the higher yeast load of 3.9×105 ± 5.51 CFU/g and the samples treated under indirect sun-drying conditions recorded a lower count of 8.6×104±10.06 cf u/g (Figure 4(a)). A significant difference (P<0.05: P=0.0007) exists in the yeast loads of the direct and indirect solar-dried samples. Yeasts are commonly known to be beneficial in the food industry, but some yeast also cause food spoilage. Some yeasts can cause fermentation in fruits by breaking down sugars into alcohol and carbon dioxide[8]. This led to a net loss of dry matter and a corresponding reduction in nutritive value[20]. The indirect solar drying method had lesser incidence of yeast load due to the enclosed nature of the drying facility preventing high contamination with yeasts since, the “seeds,” called spores of yeasts travel through the air. Hand washing or hygiene protocols were also observed before visiting the facility. All these activities contribute to the low level of yeast count and reducing the incidence of dry matter and nutrient reduction in the indirect sun-dried sample (Figure 4).
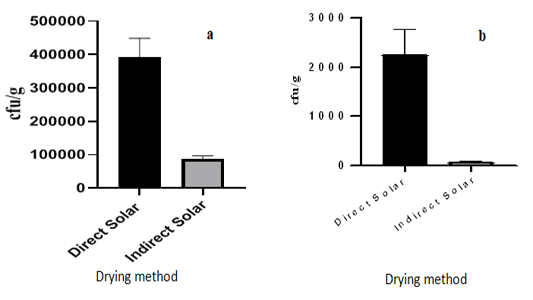
Figure 4: Effects of drying method on counts of (a) Yeast and (b) Mold of the plantain flour
The mould quantitative assay also reflected a similar trend as observed in the yeast assay with the direct solar-dried samples recording a higher mould count of 2.2×103 ± 5.03 cfu/g, where as the indirect solar-dried samples recorded 6.6×101 ± 5.27 cfu/g. A significant difference (P<0.05: P=0.0016) exists in the mould counts of the two samples. The significantly lower mould count in the indirect sun-dried sample could be attributed to the enclosed nature of the drying facility in comparison with the direct sun-drying, which was in an open space condition. In similitude with yeast counts, the spores of mould also float and travel via air and could settle on anything or space available. Hence, drying made in the open sun is susceptible to mould contamination. The period before the rainy season with unstable weather conditions during which the experiment was conducted may have also had an impact on the direct sun drying method. This probably slowed down the drying process, hence, presenting relatively high moisture sample favoring mould activity. This translated into the higher mould infection in the direct sun drying method since moulds are known to survive or thrive well in a moist or humid environment. Moldiness is one of the primary triggers for disease and health condition such as asthma, respiratory infection and cancer due to mycotoxin production[17]. Therefore, the use of indirect solar (sun) drying method is appropriate for drying as it is associated with less contamination relative to the direct sun drying.
Mold isolates from the plantain flours
The moulds isolated from the plantain flours are presented in Table 2 and Plate 2. The morphological properties of the mould isolates indicated the dominant species of moulds present to be Aspergillus niger and Aspergillus flavus. This observation corroborates the observation of Okafor and Eni (2018)[9] and Omohimi et al. (2017)[21,22] who reported Aspergillus flavus and Aspergillus niger as the most commonly isolated fungi with Aspergillus flavus as the most prevalent trailed by Aspergillus niger. However, an opposing observation was made by Ajayi and Olorundare (2014)[22], stating that Aspergillus niger is the most prevalent trailed by Aspergillus flavus. Geographical difference and sample variety could be responsible for this opposing observation. Amongst these two species of Aspergillus, flavus is typically associated with the production of mycotoxins (Aflatoxins A) which are detrimental to human health and are of public health concern (Plate 2).
Table 2: Identification of moulds using macroscopic morphology
|
Sample |
Species |
|
Direct Solar Drying |
Aspergillus flavus |
|
Indirect Solar Drying |
Aspergillus niger, Aspergillus flavus |
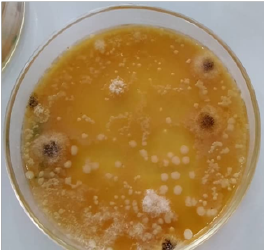
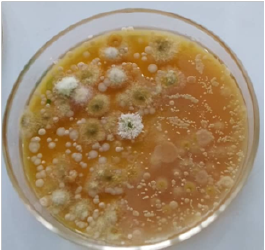
Plate 2: Molds and yeast isolates (mixed) from the plantain flour samples
The aflatoxin-bearing fungi identified on the direct and indirect solar-dried plantain flour samples must have been present in the surrounding air as spores settling on the plantain samples during the drying process. Because of this, this study also quantified the aflatoxin concentration in the dried plantain flour samples.
Effect of drying method on mycotoxin contents
The concentration of aflatoxins in the two plantain flour samples as determined in this study is shown in Table 3. The aflatoxin concentrations of the samples ranged between 4.7 – 5.4 ppb. The indirect solar-dried plantain flour sample had significantly lower (p<0.05) aflatoxin level of 4.75 ppb in comparison with 5.35 ppb of the direct solar-dried plantain flour sample. However, these concentrations of aflatoxins recorded in this study were within the acceptable aflatoxin limit of 15 ppb(µg/kg) established by the Codex Alimentarius Commission. This implies that the flour samples are safe for human consumption. Yet, it is recommended for good manufacturing processes and good storage as well as cooking practices be adopted not to encourage mycotoxins infections as their toxicity accumulates over time, presenting long term effect than short term[23]. The level of aflatoxins recorded in this study verifies that reported by Okafor and Eni (2018)[9] for flour samples.
Table 3: Aflatoxin Concentration* for Indirect Solar Dried Sample
|
Drying method |
Aflatoxin (ppb) |
|
ISD |
4.75 ± 0.050b |
|
DSD |
5.35 ± 0.050a |
*Data is mean ± sem (n=3). Means with a different superscript in the same column are significantly different (p<0.05). ISD, indirect solar drying; DSD, direct solar drying.
The direct solar-dried plantain flour sample produced a lot of mould as a result of high moisture content but did not reflect in the production of aflatoxin. Perdoncini et al. (2019)[24], reported that high levels of mould contamination on a product do not necessarily mean there are high levels of mycotoxin production. Certain factors, such as nutrient availability or stress, triggers the production of toxic substances as a mode of survival[24]. Furthermore, it is possible not all available A. flavus may have produced mycotoxins as research has shown that not all A. flavus strains produce aflatoxins[25-29].
Conclusion
The study showed that the drying method had a significant impact on the physicho-chemical composition of dried plantain flour samples. Generally, the indirect solar drying method proved efficient for drying and nutritional as well as quality characteristics of the dried plantain flour samples. Regardless of the presence of moulds, the mycotoxins levels detected in the flour sample were below the aflatoxin limit established by the Codex Alimentarius Commission (CAC). There could be other mycotoxins which were not considered in this study and as such require further study to detect their presence or otherwise. Good agricultural practices (GAP), good manufacturing processes (GMP) and good storage as well as cooking practices coupled with proper personal hygiene processes (PHP) be adopted not to encourage mycotoxins infections as their toxicity accumulates over time presenting long term ill-health.
References
- 1. Encyclopaedia, B. Plantain. (2019) In Britannica Concise Encyclopedia: Britannica Digital Learning.
Pubmed | Crossref | Others
- 2. Cafasso, J., Pletcher, P. Nutrition Facts and Health Benefits. (2016) [Plantain].
Pubmed | Crossref | Others
- 3. Amaya, N. The World’s Leading Plantain Producers.
Pubmed | Crossref | Others
- 4. Loos, T.K., Hoppe, M., Dzomeku, B.M., et al. The Potential of Plantain Residues for the Ghanaian Bioeconomy—Assessing the Current Fiber Value Web. (2018) Sustainability 10(12): 4825.
- 5. Yarkwan, B., Uvir, R.H. Effects of Drying Methods on the Nutritional Composition of Unripe Plantain Flour. (2015) Food Science and Quality Management 41: 5-11.
Pubmed | Crossref | Others
- 6. Sabarez, H. Drying of Food Materials-CSIRO Food and Nutrition. In.Stahl, A. (2014). 11 Plant-food processing: implications for dietary quality. (2016) Foraging farming 31: 171.
Pubmed | Crossref | Others
- 7. Brennan, J.G. Theory of Air-drying. (2003) In.
Pubmed | Crossref | Others
- 8. Norman, W.D., Singh, R.P. Food preservation. (2018)
Pubmed | Crossref | Others
- 9. Okafor, S.E., Eni, A.O. Microbial Quality and the Occurrence of Aflatoxins In Plantain/Yam And Wheat Flours In Ado-Odo Ota. (2018) IOP Conference Series: Earth and Environmental Science 210: 012017.
- 10. Golly, M.K., Amadotor, B. Nutritional composition of the seed of Icacina senegalensis/oliviformis (false yam). (2013) Parkistan Journal of Nutrition 12(1): 80-84.
- 11. Mold. Toxins, Mycotoxins, Endotoxins, (2012) Fusariotoxin.
Pubmed | Crossref | Others
- 12. Jackson, L.S., Al-Taher, F. Factors affecting mycotoxin production in fruits. (2008) In Mycotoxins in fruits and vegetables 75-104.
- 13. Esmaeilishirazifard, E., Keshavarz, T. Aflatoxin occurrence. Aflatoxins-Food Sources, Occurrence and Toxicological Effects. Ed. AG Faulkner, Nova Publisher, (2014) New York, USA, 35-63.
Pubmed | Crossref | Others
- 14. Villers, P. Aflatoxins and safe storage. (2014) Frontiers in microbiology 5: 158.
Pubmed | Crossref | Others
- 15. Rossiter, S. IFIS. (2019) Aflatoxins in food: Overview and definitions.
Pubmed | Crossref | Others
- 16. Dzomeku, B., Dankyi, A.A., Kodjo, D. Socioeconomic importance of plantain cultivation in Ghana. (2011) J Animal and Plant Sci 21: 269-273.
Pubmed | Crossref | Others
- 17. Mulokozi, G., Svanberg, U. Effect of Traditional Open Sun-Drying and Solar Cabinet Drying on Carotene Content and Vitamin a Activity of Green Leafy Vegetables. (2003) Plant foods for human nutrition 58: 1-15.
- 18. Golly, M.K., Samson, S.P., Charles, M.R.F. Resistance of bacteria isolates from cabbage (Brassica oleracea), carrots (Daucus carota) and lettuce (Lactuca sativa) in the Kumasi Metropolis of Ghana. (2016) Int J Nutr Food Sci, 5(4): 297-303.
Pubmed | Crossref | Others
- 19. Golly, M.K., Mills-Robertson, F.C., Amponsah, A.S., et al. Evaluation of Microbiological Contaminants of Vegetal Produce Cultivated on Animal-Manure-Enriched Soils. (2019) IOSR Journal of Environmental Science, Toxicology and Food Technology, 13(3): 20-29.
Pubmed | Crossref | Others
- 20. Ahmed, N., Singh, J., Chauhan, H., et al. Different Drying Methods: Their Applications and Recent Advances. (2013) International Journal of Food Nutrition and Safety 4(1): 34-42.
Pubmed | Crossref | Others
- 21. Adepoju, O.T., Sunday, B.E., Folaranmi, O.A. Nutrient composition and contribution of plantain (Musa paradisiacea) products to dietary diversity of Nigerian consumers. (2012) African Journal of Biotechnology 11(71).
- 22. Agoreyo, B., Akpiroroh, O., Orukpe, O., et al. The effects of various drying methods on the nutritional composition of Musa paradisiaca, Dioscorea rotundata and Colocasia esculenta. (2011) Asian Journal of biochemistry 6(6): 458-464.
- 23. Lowes, K., Shearman, C., Payne, J., et al. Prevention of yeast spoilage in feed and food by the yeast mycocin HMK. (2000) Appl Environ Microbiol 66(3): 1066-1076.
- 24. Jill, D. How to Detox Your Body from Mold Using the Low-Mold Diet (2015).
Pubmed | Crossref | Others
- 25. Omohimi, C.I., Piccirillo, C., Roriz, M., et al. Study of the proximate and mineral composition of different Nigerian yam chips, flakes and flours. (2017) J Food Sci Technol 55(1): 42-51.
- 26. Ajayi, A.O., Olorundare, S.D. Bacterial and Fungi Species Associated with Yam (Dioscorea rotundata) Rot at Akanugba-Akoko, Ondo State, Nigeria. (2014) Applied Science Research Journal 2: 12-28.
Pubmed | Crossref | Others
- 27. Perdoncini, M.R.F.G., Sereia, M.J., Scopel, F.H.P., et al. Growth of Fungal Cells and the Production of Mycotoxins. (2019) In Cell Growth: Intech Open.
Pubmed | Crossref | Others
- 28. AOAC. Official Methods of Analysis 934.01, 988.05, 620.39, 946.05, 962.09 (17th Edn. ed.). (AOAC) Association of Official Analytical Chemists, (2002) Arlington, VA, Washington DC, USA.
Pubmed | Crossref | Others
- 29. Mold. Toxins, Mycotoxins, Endotoxins, (2012) Fusariotoxin.
Pubmed | Crossref | Others
- 30. WHO. (2018) Mycotoxins.
Pubmed | Crossref | Others












