Effects of Adjuvant in Potentiating the Analgesic Effect of Fascia Iliaca Compartment Block
Ahmed Zein#
Affiliation
- Departments of Anesthesiology and I.C.U Al-Minia University, Faculty of Medicine, Egypt
- #All author’s contribution to the paper are about equal
Corresponding Author
Josef Zekry Attia, MD, Ph.D, Department of Anesthesiology, Faculty of Medicine, Minia University, 61111, Minia, Egypt, Tel: +201000427407, Fax: +20862324414; E-mail: josefzekry2@yahoo.com
Citation
Attia, Z.J., Zein, A. Effects of Adjuvant in Potentiating the Analgesic Effect of Fascia Iliaca Compartment Block. (2017) J Anesth Surg 4(2): 86- 92.
Copy rights
© 2017 Attia, Z.J. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Bupivacaine, Dexmedetomidine, Ketamine, Fascia iliaca compartment block (FICB)
Abstract
Objective: Nerve blocks for postoperative analgesia have increased significantly lately. Beside the use of adjuvants plus local anesthetics in nerve block in order to extend the analgesia time.
Materials and Methods: Sixty elderly patients, ASA grades II and III scheduled for surgery after hip fracture under intrathecal anesthesia with pre-operative FICB, The patients were blindly randomly allocated into three-study groups, all of them received preoperative FICB “bupivacaine 0.25 % with adjuvant either Group K received ketamine (50 mg), Group D received Dexmedetomidine (100 μg) and Group KD received ketamine (50 mg) + Dexmedetomidine (100 μg) plus intrathecal block.
Results: Group “KD” showed more satisfactory during positions to receive intrathecal block in compare with either groups due to significant rapid onset of preoperative analgesia in compared with other groups, group KD show significant rapid onset of sensory and motor block, prolonged duration of sensory block, and postoperative analgesia as compare with both group with significant decrease in total analgesic requirement. While group D provided significant prolonged duration of sensory block, and postoperative analgesia when compare with group K with significant decrease in total analgesic requirement. There were no significant difference between the three groups, hemodynamic change (heart rate and blood pressure) and side effects in form of baradycardia, hypotension, nausea, vomiting and sedation.
Conclusion: The addition of ketamine, dexmedetomidine with bupivacaine 25% in FICB, is considered the best combination for intrathecal anesthesia for hip repair surgery in elderly patients. It provided intraoperative hemodynamic stability, prolonged motor and sensory block as well as potentiating the postoperative analgesic effect with minimal complications.
Introduction
Recently, nerve blocks for postoperative analgesia has increased significantly, with using of adjuvants plus local anesthetics which extends the analgesia time[1]. A wide variety of agents have been tested as adjuvant to nerve blocks, which are commonly performed for regional anesthesia, for example opioids (acting in peripheral kappa receptors)[2], midazolam( via acting on peripheral GABA receptors)[3] naloxone[4] dexmedetomidine (has synergistic effect in nerve block and it has a high binding affinity to nerve fiber α²-adrenoreceptors)[5,6] clonidine (binding affinity to nerve fiber α²-adrenoreceptors)[7], epinephrine (induced vasoconstriction with decrease absorption of local anesthetic)[8], Ketamine (via acting as noncompetitive antagonist of the N-methyl-D aspartate receptor (NMDAR)[9] and recently dexamethasone (may be through its proinflammatory effect)[10]. These drugs have been used along with local anesthetics agents for this purpose with varying degrees of success.
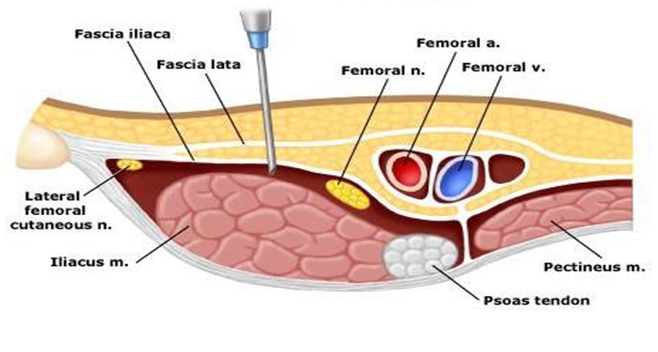
The fascia iliaca compartment is a potential space with the following limits anteriorly: the posterior surface of the fascia iliaca, which covers the iliacus muscle posteriorly: the anterior surface of the iliacus muscle and the psoas major muscle. Medially: the vertebral column. Cranio-laterally: the inner lip of the iliac crest, and cranio-medially: it is continuous with the space between the quadrates muscle and its fascia. This compartment allows deposition of local anesthetic of sufficient volumes so as to spread to at least two of the three major nerves to the femoral and lateral femoral cutaneous nerves[11]. (Figure 1)
Figure 1:
This study was conducted to evaluate the efficacy of adding ketamine to Dexmedetomidine and bupivacaine in FICB in elderly patients undergoing hip repair surgery.
Materials and Methods
After institutional Ethics Committee approval, this prospective double-blind, randomized, controlled study was conducted at the department of anesthesia and intensive care unit, El- Minia University Hospital from May 2016 to March 2017. The study involved sixty adult patients above 60 years of both sexes, ASA grades II and III scheduled for surgery after hip fracture under intrathecal anesthesia with pre-operative FICB. All patients gave written informed consent.
Exclusion criteria included patients refuse to joint this study, contraindication of intrathecal anesthesia, and any contraindication of regional block (FICB). The routine investigations were done to all patients; the drugs were prepared in the same volume (30 ml) outside the operating room by an assistant not involved in the procedure. The name of the drug to be given was sealed in envelopes numbered 1 - 60. A coded form of drug was then convey to the another anesthesiologist who was blind to the type of drug given. The drugs used were Dexmedetomidine (precedex 200 mcg / 2 ml [100 mcg/ml] Hospira, Inc, Lak Forest, USA) and ketamine 50 mg / 1 ml, and bupivacaine 0.25% (2.5 ml / ml diluted with normal saline).
The patients were randomly allocated into three-study groups of 20 patients each as per computer-generated random number list:
Group K received: intrathecal 7.5 mg of 0.5% hyperbaric bupivacaine (1.5 ml) + received 29 ml of 0.25% bupivacaine (total 72.5 mg) with 1 ml ketamine (50 mg).
Group D received: intrathecal 7.5 mg of 0.5% hyperbaric bupivacaine (1.5 ml) + received 29 ml of 0.25% bupivacaine (total 72.5 mg) with 1 m (100 μg) Dexmedetomidine.
Group KD: intrathecal 7.5 mg of 0.5% hyperbaric bupivacaine (1.5 ml) + received 28 ml of 0.25% bupivacaine (total 70 mg) with 1 ml (100 μg) ketamine 1 ml + Dexmedetomidine (50 mg).
We instructed the patients preoperatively about visual analog scale (VAS) (0 for no pain, 10 for the worst pain) and modified bromage block (“0” for no paralysis, “3” for loss movement)[12] to measure the peri-operative analgesia and anesthesia. After an intravenous access was established, 500 ml of acetated Ringer’s solution was infused. An ultrasound – guided FICB block was performed before intrathecal block to facilitate patients positioning for receiving intrathecal block. Skin was prepared with 2% chlorhexidine solution. Infiltrate the skin superficially with 1 - 2 mls of 1% lignocaine at this point of injection and a high frequency (11 - 6 MHz) linear ultrasound probe (2.5 cm footprint) (chison ECO3, chisonInc) was used, used syringes were prepared under aseptic technique. Ultrasound probe was positioned in injected point that identified by located two bone provenance “ASIS and pubic tuberosity”, then Locate the femoral pulse, femoral nerve is just lateral to arterial pulsation, the intended injection site is 1.5 - 2 cm lateral to pulsation, A 100 mm long 20G short bevel needle (Stimuplex A B/BRAUN Melsungen AG, Germany) was inserted in plane to the probe of the ultrasound anteriorly. Needle pierces the skin at right angles. Keep the needle in the sagittal plane so as to avoid the neurovascular bundle which lies medially. Advance the needle through two distinct “pops” as it perforates first the fascia lata and then the fascia iliaca. Advance the needle a further 1 - 2 mm. Aspirate, and if negative inject slowly. Successful injection was obtained when an echoluescent lens-shape appeared between fascia iliaca and iliacus muscle.
Non-invasive blood pressure, ECG and peripheral oxygen saturation were monitored using a multi-parameter monitor (Mindray iMEC12, Hi-tech industrial Park, Nanshan, Shenzhen, china). All patients were assessed for easy positioning for receiving intra thecal block.
Pain assessment was done by VAS score and motor power assessed with modified bromage score ever 5 min from block. Then after 30 min from FICB and under all aseptic precautions in sitting position, before performance intrathecal injection, Assessment of position to receive intrathecal block patients satisfaction assessed between excellent, good, fair and poor among the three groups of patients then a median spinal puncture was performed at the L3-L4 interspinous spaces using a 22 gauge Quincke type point spinal needle. Then, the patients were placed in the supine position with elevation of head 15 degree. Oxygen was continuously given to the patients via a nasal cannula. Mean blood pressure (MBP) and heart rate (HR) were recorded every 5 min up to 15 min and then every 15 min up to 90 min.
After intra thecal block, the assessment of sensory level of block and motor power were done by using the loss of sensation to pinprick and by modified bromage scale for muscle power every 2 minutes, the surgeons started the procedures when the block level was T10 and modified bromage scale 3.
1. Time of onset of analgesia: it was the time from drug injection in FICB to the VAS ≥ 3 with pinprick at cutaneous area supply by femoral nerve and fracture area.
2. The time of onset of sensory block: it was the time from drug injection in intrathecal to the loss of sensation “ VAS > 3 “ to pinprick at T10 level after intra thecal block and the Peak block level was the level that persists during four consecutive tests.
3. The time of onset of motor block: it was the time from drug injection in FICB to the modified bromage block scale = 3 , that persists during four consecutive tests.
4. Duration of sensory block: it was the time from intrathecal injection to two segment regression.
5. Duration of motor block: it was the time from in thecal injection to modified bromage scale 1 (flexion of hip).
6. The time of postoperative analgesia : it was the time from end of surgery to the VAS ≤ 3 with pinprick.
7. 24 hs total nalbuphine analgesia requirement: with VAS ≤ 3, nalbuphine was applies 5 mg IV “the amount of applied nalbuphine calculated within 24 hs”.
In case the block failed to reach the level of T10 within 20 min of intrathecal injection, the intrathecal dose was considered as inadequate and excluded from the study[13]. Further assessment of pain and motor power was then performed every 1hs in the recovery room until bromage score returned to zero (the duration of motor block).
Statistical Analysis
Data were represented as means ± standard errors of the mean (SEM). Statistical analysis was performed using Graph pad Prism 5 software using one-way ANOVA followed by Tukey-Kramar post hoc for comparison between groups with a value of P < 0.05 considered statistically significant. The sample size was calculated using power analysis (α = 0.05, β = 0.8) was found to require at least 18 patients per group. Thus, we decided to include 20 patients per group to allow for possible drop-out.
Results
Sixty elderly patients undergoing hip repair surgery under preoperative FICB with intrathecal block, with whom we completed this study.
Pre-operative facilitate positioning: As regard satisfaction of patient in positioning, patients in group KD provided excellent satisfactory “more comfortable in positing even in present of fracture hip”, more than group D, and K, on other hand group K provide the worst satisfaction in compare with other groups (Table 1).
Table 1: Patient’s satisfaction in the three groups at pre-operative facilitate positioning of patients.
| Patient satisfaction | Group K N = 20 | Group D N = 20 | Group KD N = 20 |
|---|---|---|---|
| Excellent | 5 (25%) | 12 (60%) | 15 (75%) |
| Good | 10 (50%) | 6 (30%) | 4 (20%) |
| Fair | 4 (20%) | 2 (10%) | 1 (5%) |
| Poor | 1(5%) | - | - |
• Data are presented as number (%).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (ketamine + dexmedetomidine).
Demographic data: There were no significant changes a regard Patients’ demographic data in the three groups (Table 2).
Table 2: Demographic data (patient characteristics).
| Group K N = 20 | Group D N = 20 | Group KD N = 20 | P Value | |
|---|---|---|---|---|
| Age (years) | 75.63 ± 0.33 | 78.57 ± 0.23 | 79.15 ± 0.29 | 0.7324 |
| Weight (kg) | 76.67 ± 0.49 | 79.53 ± 0.60 | 77.83 ± 0.87 | 0.4584 |
| Height (cm) | 167.3 ± 0.42 | 165.0 ± 0.36 | 169.3 ± 0.33 | 0.8777 |
| Gender (male/female) | 8/12 | 9/11 | 7/13 | |
| Duration of surgery (min) | 107.7 ± 042 | 100 ± 0.42 | 100.8 ± 8.31 | 0.6326 |
• Data represent mean ± SE.
• One way ANOVA test for parametric quantitative data between the three Group followed by Turkey multiple comparison test at (P value < 0.05).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (ketamine + dexmedetomidine).
Time of onset of analgesic effect of FICB: As regard time of onset of analgesic there were significant decrease in time onset of preoperative analgesia in group KD when compared with group K, and D, on the other hand there were significant decreases in time onset of analgesia in group D when compared with group K (Table 3).
Table 3:
| Onset of analgesia VAS > 3 (min) | |
|---|---|
| Group K N = 20 | 33.86 ± 0.91 |
| Group D N = 20 | 27.14 ± 0.51 |
| Group KD N = 20 | 14.57 ± 0.57 |
| P value (group K versus group D) | P < 0.001 |
| P value (group K versus group KD | P < 0.001 |
| P value (group D versus group KD | P < 0.001 |
• Data represent mean ± SE.
• One way ANOVA test for parametric quantitative data between the three Groupfollowedby Turkey multiple comparison test at (P value < 0.05).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (ketamine + dexmedetomidine).
Time onset of sensory and motor block: As regard time of onset of sensory block there were significant decrease time onset of sensory block in group KD when compared with group K, and D, on the other hand there were significant decreases time onset of sensory block in group D when compared with group K (Table 4 ). As regard time onset of motor block, there were significant decrease time onset of motor block in group KD when compared with group K, while insignificant change when compared with group D, on the other hand there were significant decreases time onset of sensory block in group D when compared with group K (Table 4 ).
Table 4: Effect of ketamine, Dexmedetomidine and both of them on onset of sensory and motor block during FICB.
| Onset of sensory block (min) | Onset of motor block (min) | |
|---|---|---|
| Group K N=20 | 6.1 ± 0.26 | 7.2 ± 0.29 |
| Group D N=20 | 4.57 ± 0.29 | 5.3 ± 0.26 |
| Group KD N=20 | 2.7 ± 0.28 | 5.9 ± 0.23 |
| P value (group K versus group D) | P < 0.01 | P < 0.01 Significant |
| P value (group K versus group KD | P < 0.01 | P < 0.01 Significant |
| P value (group D versus group KD | P < 0.01 | P < 0.01 In-significant |
• Data represent mean ± SE.
• One way ANOVA test for parametric quantitative data between the three group followed by Turkey multiple comparison test at (P value < 0.05).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (ketamine + dexmedetomidine)
Duration of sensory and motor block: As regard duration of sensory and motor block, there was significantly prolonged time of sensory block in KD when compared with group K and D, on the other hand there were prolonged in time of sensory block in group D when compared with group K (Table 5). As regard duration of motor block, there was significantly prolonged time of motor block in KD when compared with group K and D, on the other hand there were prolonged in time of motor block in group D when compared with group K (Table 5).
Table 5: Effect of ketamine, Dexmedetomidine and both of them on duration of sensory and motor block during FICB.
| Duration of sensory block (min) | Duration of motor block (min) | |
|---|---|---|
| Group K N = 20 | 182. 14 ± 0.71 | 164.8 ± 13.99 |
| Group D N = 20 | 213.9 ± 12.97 | 181.6 ± 12.14 |
| Group KD N = 20 | 301.6 ± 11.92 | 205.2 ± 12.65 |
| P value (group K versus group D) | P < 0.001 | P < 0.001 |
| P value (group K versus group KD | P < 0.001 | P < 0.001 |
| P value (group D versus group KD | P < 0.001 | P < 0.001 |
• Data represent mean ± SE.
• One way ANOVA test for parametric quantitative data between the three Group followed by Turkey multiple comparison test at (P value < 0.05).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (dexmedetomidine + ketamine).
Post operative analgesia: Mean time postoperative analgesia (when patient were VAS > 3 and need additional analgesia) there was significantly prolonged time of analgesia in KD when compared with group K and D, on the other hand there were prolonged in time of analgesia in group D when compared with group K (Table 6).
Table 6: Effect of ketamine, Dexmedetomidine and both of them on time to first postoperative analgesia during FICB.
| Time to first postoperative analgesia (min) | |
| Group K N = 20 | 386.0 ± 10.5 |
|---|---|
| Group D N = 20 | 540.1 ± 19.4 |
| Group KD N = 20 | 789.1 ± 2 6.4 |
| P value (group K versus group D) | P < 0.001 |
| P value (group K versus group KD | P < 0.001 |
| P value (group D versus group KD | P < 0.001 |
• Data represent mean ± SE.
• One way ANOVA test for parametric quantitative data between the three group followed by Turkey multiple comparison test at (P value < 0.05).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (dexmedetomidine + ketamine ).
Total amount nalbuphine requirement: As regard total amount of nalbuphine required in 24 h, there was significantly decrease in analgesic requirement in KD group when compared by group K and D group, on the other hand decrease in group D when compared with group K (Table 7).
Table 7: Effect of ketamine, Dexmedetomidine and both of them total amount nalbuphine requirement during FICB.
| Total amount of nalbuphine in mg /24h | |
|---|---|
| Group K N = 20 | 9.4 ± 0.37 |
| Group D N = 20 | 6.4 ± 0.24 |
| Group DK N = 20 | 4.11 ± 0.35 |
| P value (group K versus group D) | P < 0.001 |
| P value (group K versus group KD | P < 0.001 |
| P value (group D versus group KD | P < 0.001 |
Hemodynamic changes: There was no significant difference between the three groups regarding the hemodynamic changes (heart rate and blood pressure).
Incidence of complication: Concerning the incidence of side effects e.g bradycardia and hypotension, nausea, vomiting and sedation, it was mentioned in (Table 8).
Table 8: Incidence of side effects.
| Group K N = 20 | Group D N = 20 | Group KD N = 20 | |
|---|---|---|---|
| Bradycardia | 1 (5%) | 2 (10%) | 2 (10%) |
| Hypotension | 1 (5%) | 4 (20%) | 5 (25%) |
| Nausea & Vomiting | 2 (10%) | 1(5%) | 4 (20%) |
| Sedation | 2.8 ± 0.8 | 0.8 ± 0.7 | 2.9 ± 0.7 |
• Data are presented as number (%).
• Group K (ketamine), Group D (dexmedetomidine) and Group KD (ketamine + dexmedetomidine). DHS: Dynamic Hip Screw.
Discussion
Fascia iliaca compartment block has three main advantages. Firstly, it results in fewer hemodynamic changes. Secondly, fascia iliaca compartment block may be performed while the patient is supine, Thirdly, the risk of nerve injury is less and nerve stimulation is not needed[14].
Also, Pre operative application of FICB provide two more advantage in addition to its recorded advantages, first: that it provide analgesia make patient more comfortable in position to receive intrathecal injection (With respect to positioning of patients with hip fracture, which is so difficult to put the patient in proper position for intrathecal block, and it causes severe pain that result in severe tachycardia and hypertension “pain-stress response stimulate sympathetic or bradycardia and hypotension” “pain stimulate neurogenic shock parasympathetic”). Secondly: also allowed use small amount of local anesthetic drugs intra-thecal with minimal hemodynamic changes, especially in orthopedic surgery with elder patients, those can be progress to many complication if there hemodynamic instability or weak analgesia during surgery.
On the other hands, use Combinations of drugs a for nerve block such as FICB, targeting multiple nerve fiber receptors lead to prolonged analgesia with superior quality, which can be achieved by relatively small volumes of individual drugs with subsequent decreased incidence of nerve fiber compression[15]. These approved in our study especially with use of adjuvant such as combination of ketamine and Dexmedetomidine this mixture prolongs the duration of the sensory block, with decrease total nalbuphine requirement. These combinations provide a considerable effect with producing minimal hemodynamic changes with improvement in the patient satisfaction.
Ketamine reduces excitatory NMDA mediated neurotransmission in interneuron, leading to a decrease in the excitability of spinal dorsal horn neurons. It absorption into circulation and can result in release of an endogenous opioid that actsat spinal delta receptor. Thus, it can potentiate the effect of bupivacaine andenhance the intra-operative anesthesia and analgesia in addition to postoperative analgesia[16].
Lashgarinia et al[17], Tverskoy et al[18], Dowdy et al[19] and Weber et al[20] there result confirm the potential analgesic effect of ketamine as adjuvant to nerve block.
Lashgarinia et al[17] observed that while addition of ketamine (2 mg/kg) to (5 mg/kg) lidocaine 1.5 % in supra clavicular block to sixty adult patients undergoing elective surgery of the elbow, forearm, wrist or hand, resulted in a low VAS, at all-time points during the first 24 hours after surgery. The time of first request for analgesia in the ketamine group was significantly more than that in the control group. Tverskoy et al[18] observed that ketamine acting via a peripheral mechanism can profoundly enhance anesthetic and analgesic actions of a local anesthetic administered for infiltration anesthesia.
Local anesthetic properties of ketamine were demonstrated by Dowdy et al[19] who reported that ketamine could produce reversible inhibition of the mixed action potential in the stimulated frog sciatic nerve. Also, dogs injected with ketamine rapidly developed reversible segmental paralysis with no alteration inconsciousness state. In addition the effect of ketamine on nerve conduction was confirmed by Weber et al[20] who reported that the subcutaneous infiltration of ketamine caused a loss of thermal and pain sensations for eight to ten minutes.
Also Webeer et al[20,21] suggest that the effect of ketamine is more likely to occur locally in an inflamed tissue, but not at the level of a nerve plexus distant from the surgical site. Ketamine has demonstrated a significant anti-inflammatory effect that significantly inhibits the early postoperative inflammatory response. By acting at different levels of inflammation, interacting with inflammatory cell recruitment, cytokine production, and inflammatory mediator regulation.
On the other hand, Rahimzadehet al[22], Lee et al[23] disagree with the previous finding, there results were deny any potential analgesic effect of ketamine on local nerve bock. Rahimzadehet al[22] reported that sixty patients, ASA I, II that underwent elective knee surgery for repairing anterior cruciate ligament under spinal anesthesia were randomly allocated to receive either ropivacaine 0.2% or an equivalent volume of ropivacaine 0.1% plus 1.0 mg/kg ketamine via continuous femoral block with pump infusion. VAS differed at various time points after surgery, with higher scores in patients who received concomitant ketamine and ropivacaine. The degree of quadriceps muscle weakness was similar between the groups at the different time points. Patients in ropivacaine group showed better quality of pain control with appropriate sedation in comparison with the patients in ketamine/ropivacaine group.
Lee et al[23] study carried on Sixty adults patients scheduled for forearm or hand surgery under the interscalene brachial plexus block were prospectively randomized to receive 0.5% ropivacaine 30 ml with 30 mg ketamine. This study suggests that 30 mg ketamine added to ropivacaine in the brachial plexus block does not improve the onset or duration of sensory block, but it does cause a relatively high incidence of adverse-effects. These two findings do not encourage the use of ketamine with local anesthetics for brachial plexus blockade.
On the other hand, The prolonged sensory and motor blockade resulted from the addition of Dexmedetomidine, is not well understood but Niesters M[24] study explain the analgesic effect of dexmedetomidine is related to the interference with neuromuscular activity, or through binding of α² agonists to motor neurons in the dorsal horn “after its absorption in systemic circulation” or via the additive or synergistic effect to the local anesthetics. Calasans-Maia JA[25] explain action of dexmedetomidine on dorsal horn cell by inhibiting the release of C-fiber transmitters, glutamate and substance P from the terminals of primary afferent and by G-protein mediated activation of K+ channels which induce hyperpolarization of postsynaptic dorsal horn neurons whereas bupivacaine acts as a local anesthetic by blocking Na+ channels. This explains synergism effect of Dexmedetomidine with local anesthetic.
Consequently, the addition of dexmedetomidine to bupivacaine produced a faster onset and longer duration of sensory block as well as a prolonged postoperative analgesia with minimal hemodynamic effects compared to bupivacaine alone[26,27,28]. Capdevila et al[29] and Calasans-Maia et al[25] there result confirm the potential analgesic effect of Dexmedetomidine. Capdevila et al[29] proved that addition of Dexmedetomidine to local anesthesia in FICB will reduce the volume of local anesthesia requirement to achieve adequate effect. Furthermore, brown et al proved the same result[30].
Calasans-Maia et al[25] found that Dexmedetomidine prolongs spinal anesthesia induced by levobupivacaine 0.5% inguinea-pigs. The same result proved by Esmaogluet al as well[31] by addition of Dexmedetomidine to lidocaine for intravenous regional anesthesia. Our result, recorded more benefited by using combination of ketamine and Dexmedetomidine in FICB these combination improve perioperative analgesia with menial side effect.
Conclusion
The addition of preoperative ketamine, dexmedetomidine with 0.25% bupivacaine in FICB to intrathecal block, is considered better combination for hip repair surgery in elderly patients. It provides intraoperative hemodynamic stability, prolonged motor and sensory block as well as potentiating the postoperative analgesic effect with minimal complications.
Highlights: It is first time to use ketamine with Dexmedetomidine as an adjuvant in single injection in nerve block. We need further studies for nerve tissue safety with in injection of large volume.
Acknowledge:
We thank all member of anesthesiology department, faculty of medicine. Minia University, Egypt.
Conflict of interest:
The authors declare that there is no conflict of interest.
Funding:
This research received no specific grant from any funding agency in the public.
References
- 1. Kirksey, M.A., Haskins, S.C., Cheng, J., et al. Local Anesthetic Peripheral Nerve Block Adjuvants for Prolongation of Analgesia: A Systematic Qualitative Review. (2015) PLoS One 10: e0137312.
Pubmed || Crossref || Others - 2. Bailard, N.S., Ortiz, J., Flores. R.A., et al. Additives to local anesthetics for peripheral nerve blocks: Evidence, limitations, and recommendations. (2014) Am J Health Syst Pharm 71(5): 373-385.
Pubmed || Crossref || Others - 3. Trivedi, V., Patel, N.A. Comparative clinical study of injection clonidine versus midazolam in supraclavicular brachialplexus block for sedation and postoperative analgesia: a study of 60 cases. (2010) J Indian Med Assoc 108(9): 563-567.
Pubmed || Crossref || Others - 4. Movafegh, A., Nouralishahi, B., Sadeghi, M., et al. An ultra low dose of naloxone added to lidocaine or lidocaine fentanyl mixture prolongs axillarybrachial plexus blockade. (2009) AnesthAnalg 109(5): 1679-1683.
Pubmed || Crossref || Others - 5. Asano, T., Dohi, S., Ohta, S., et al. Antinociception by epidural and systemic alpha (2)-adrenoceptor agonists and their binding affinity in rat spinal cord and brain. (2000) AnesthAnalg 90(2): 400-407.
Pubmed || Crossref || Others - 6. Swami, S.S., Keniya, V.M., Ladi, S.D., et al. Comparison of dexmedetomidine and clonidine (α2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. (2012) Indian J Anaesth 56(3): 243-249.
Pubmed || Crossref || Others - 7. Nishikawa, K., Kanaya, N., Nakayama, M., et al. Fentanyl improves analgesia but prolongs the onset of axillary brachial plexusblock byperipheral mechanism. (2000) AnesthAnalg 91(2): 384-387.
Pubmed || Crossref || Others - 8. Chawda, P.M., Sharma, G. A clinical study comparing epinephrine 200 μg or clonidine 90 μg as adjuvants to local anaesthetic agent in brachial plexus block via supraclavicular approach. (2010) JAnaesthesiol Clin Pharmacol 26(4): 523-527.
Pubmed || Crossref || Others - 9. Kristensen, J.D., Svensson, B., Gordh, T. "The NMDA-Receptor Antagonist CPP Abolishes Neurogenic Wind-Up Pain After Intrathecal Administration in Humans".Pain. (1992) 51: 249–253.
Pubmed || Crossref || Others - 10. Movafegh, A., Razazian, M., Hajimaohamadi, F., et al. ADexamethasone added to lidocaine prolongs axillary brachial plexus blockade. (2006) AnesthAnalg 102(1): 263-267.
Pubmed || Crossref || Others - 11. Nia Wyn Davies. Fascia Ilica Compartment block: Landmark approach guidelines for use in the emeregency department, CT3 access anesthetics morristion hospital version. (2016) 10.
Pubmed || Crossref || Others - 12. Bromage, P.R., Burfoot, M.F., Crowell, D.E., et al. Quality of epidural blockade I influence of physical factors. (1964) Br J Anaesth, 36: 342-352.
Pubmed || Crossref || Others - 13. Ramsay, M.A., Savege, T.M., Simpson, B.R., et al. Controlled sedation with alphaxalone-alphadolone. (1974) Br Med J 22(2): 656-659.
Pubmed || Crossref || Others - 14. De Visme, V., Picart, F., Le Jouan, R., et al. Combined lumbar and sacral plexus block compared with plain bupivacaine spinal anesthesia for hip fractures in the elderly. (2000) RegAnesth Pain Med 25(2): 158-162.
Pubmed || Crossref || Others - 15. Gupta, A., Kamat, H., Kharod, U., et al, Efficacy of intrathecal midazolam in potentiating the analgesic effect of intrathecal fentanyl in patients undergoing lower limb surgery. (2015) Anesth Essays Res 9(3): 379-383.
Pubmed || Crossref || Others - 16. Ho, K.M., Ismail, H. Use of intrathecal midazolam to improve perioperative analgesia: a meta-analysis. (2008) Anaesth Intensive Care 36(3): 365-373.
Pubmed || Crossref || Others - 17. Lashgarinia, M., Naghibi, K., Honarmand, A., et al. Effect of ketamine as an adjuvant in ultrasound-guided supraclavicular brachial plexus block: A double-blind randomized clinical trial study. (2014) Adv Biomed Res 3: 232.
Pubmed || Crossref || Others - 18. Tverskoy, M., Oren, M., Vaskovich, M., et al. Ketamine enhances local anesthetic and analgesic effects of bupivacaine by peripheral mechanism: a study in postoperative patients. (1996) Neurosci Lett 215(1): 5-8.
Pubmed || Crossref || Others - 19. Dowdy, E.G., Kaya, K., Gocho, Y. Some pharmacologic similarities of ketamine, lidocaine, and procaine. (1973)AnesthAnalg 52(5): 839-842.
Pubmed || Crossref || Others - 20. Weber, W.V., Jawalekar, K.S., Jawalekar, S.R. The effect of ketamine on nerve conduction in isolated sciatic nerves of the toad. (1975) Neurosci Lett 1(2): 115-120.
Pubmed || Crossref || Others - 21. Loix, S., De-Kock, M., Henin, P. The anti-inflammatory effects of ketamine: state of the art. (2011) Acta Anaesthesiol Belg 62(1): 47-58.
Pubmed || Crossref || Others - 22. Rahimzadeh, P., Faiz, S.H.R., Ziyaeifard, M., et al. Effectiveness of adding ketamine to ropivacaine infusion via femoral nerve catheter after knee anterior cruciate ligament repair. (2013)J Res Med Sci 18(8): 632-636.
Pubmed || Crossref || Others - 23. Lee, I.O., Kim, W.K., Kong, M.H., et al. No enhancement of sensory and motor blockade by ketamine added to ropivacaineinterscalene brachial plexus blockade. (2002) ActaAnaesthesiolScand 46(7): 821-826.
Pubmed || Crossref || Others - 24. Niesters, M., Martini, C., Dahan, A. Ketamine for chronic pain: risks and benefits. (2014) Br J ClinPharmacol 77(2): 357-367.
Pubmed || Crossref || Others - 25. Calasans-Maia, J.A, Zapata-Sudo, G., Sudo, R.T. Dexmedetomidine prolongs spinal anaesthesia induced by levobupivacaine 0.5% in guinea-pigs. (2005) J Pharm Pharmaco 57(11): 1415-1420.
Pubmed || Crossref || Others - 26. Eisenach, J.C., De Kock, M., Klimscha, W. Alpha(2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995). (1996) Anesthesiology 85(3): 655-674.
Pubmed || Crossref || Others - 27. Kim, J.E., Kim, N.Y., Lee, H.S., et al. Effects of intrathecal dexmedetomidine on low-dose bupivacaine spinal anesthesia in elderly patients undergoing transurethral prostatectomy. (2013) Biol Pharm Bull 36(6): 959-965.
Pubmed || Crossref || Others - 28. Chang, Y.S., Kim, J.E., Sung, T.Y. Low-dose Bupivacaine with Dexmedetomidine Prevents Hypotension After Spinal Anesthesia. (2015) Open Anesthesiology Journal 9:39-45.
Pubmed || Crossref || Others - 29. Capdevila, X., Biboulet, P., Bouregba, M., et al. Comparison of the three-in-one and fascia iliaca compartment blocks in adults: clinical and radiographic analysis. (1998) AnesthAnalg 86(5): 1039-1044.
Pubmed || Crossref || Others - 30. Brown, D.L. Atlas of regional anesthesia. (1992) Philadelphia: W.B SaundersCo 63-72.
Pubmed || Crossref || Others - 31. Esmaoglu, A., Mizrak, A., Akin, A., et al. Addition of dexmedetomidine to lidocaine for intravenous regional anaesthesia. (2005) Eur J Anaesthesiol 22(6): 447-451.
Pubmed || Crossref || Others