Electrospun Micro and Nanofibres of Olive Oil and Polyvinilic Alcohol
Gonzalez Silva, J.A1, Olguin Fuentes, M.P1, Santacruz Vázquez, C1, Toxqui López, S2, Laguna-Cortés, J.O3, Santacruz Vázquez, V1*
Affiliation
- 1Facultad de Ingeniería Química, Benemérita Universidad Autónoma de Puebla Av San Claudio, Ciudad Universitaria, Puebla, Pue México. C.P. 72570
- 2Facultad de Ingeniería, Benemérita Universidad Autónoma de Puebla Av San Claudio, Ciudad Universitaria, Puebla, Pue México C.P. 7257
- 3Departamento de Ciencias Básicas Tecnológico Nacional de México, Instituto Tecnológico de Puebla. C.P. México 72000
Corresponding Author
V. Santacruz Vázquez, Facultad de Ingeniería Química, Benemérita Universidad Autónoma de Puebla Av San Claudio, Ciudad Universitaria, Puebla, Pue México. C.P. 72570, México. E-mail: versanva@gmail.com
Citation
Santacruz Vázquez, y.v., et al. Electrospun Micro and Nanofibres of Olive Oil and Polyvinilic Alcohol. (2017) I J Food Nutri Sci 4(2): 110- 116.
Copy rights
© 2017 Santacruz Vázquez, V. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Electrospinning; Fibers; Polyvinyl alcohol; Olive oil; Encapsulation
Abstract
Electrospun micro and nanofibres were obtained from an emulsion of Polyvinyl Alcohol (PVA) at a concentration of 10% and olive oil at 4 and 8% w / w. Scanning electron microscope images showed tubular, continuous fibers, whose diameters are dependent on the concentration of the PVA and olive oil. Fourier transform infrared spectroscopy in-dicated that the obtained fibers were composed of both components. The study demonstrated the potential to encapsulate liposoluble compounds in polymeric fibers using the electrospinning technique.
Introduction
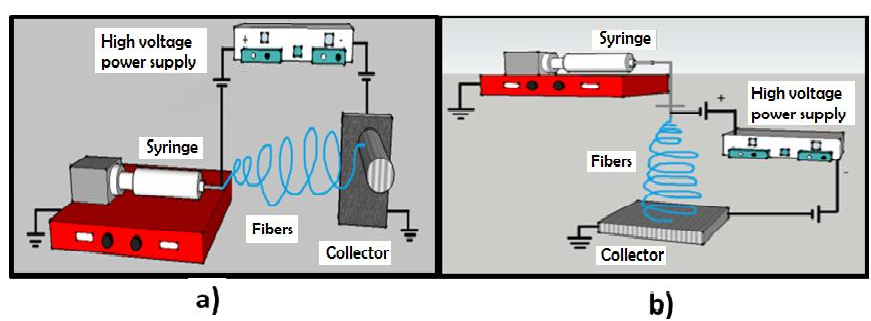
Electrospinning is a method of obtaining nanofibers[1]. The assembly for the execution this technique consists of a capillary injector, through which a polymer solution its ejected, and a high voltage source that has two electrodes, one of which must be connected to the injector and the other directly to the collecting plate where the fibers are deposited[2]. The technique can be set up horizontally or vertically (Figure 1).
Figure 1: Installation scheme for horizontal electrospinning (a) vertical electrospinning (b).
The polymer must be diluted in any solvent or solvents that allow a complete dilution thereof in Order to avoid clogging the capillary injector during the process, but at the same time promoting the Production of homogeneous fibers. The polymers used in the electrospinning process are dielectric and in the presence of an electric field, can be considered as an arrangement of microscopic electrical dipoles composed of positive and negative charges whose centers do not coincide and only change their position in response to strong external electric fields, allowing the solution stretch and form fibers[3].
It is possible to increase the dielectric properties of the solution for example by adding solvents with high dielec-tric values[4]; this favors the formation of fibers with fewer defects[5]. When the solution has been introduced into the injector and the electric potential difference applied, charges are accumulated in the drop. This then lengthens to create a shape known as a Taylor cone. When the forces due to the electric field strength exceed the cohesive forces of the solution, a jet of polymer solution is elongated due to the electrical interactions between the molecular charges in the vicinity and begins a journey from the tip of the injector to the collector plate. Along the path of the fibers, the solvent evaporates and the fibers solidify upon arrival at the collector[6]. There are numerous variables that may influence the properties and characteristics of the electrospun fibers and the control of these variables during execution of this process is of the upmost importance. The concentration of the solution is one of the parameters that effects the morphology of the fibers, since it influences the surface tension, solution viscosity and fiber diameter[7,8]. Solution conductivity is another variable that affects fiber formation through the transport of charges[1]. The addition of salts to the polymer solution increases the conductivity and consequently the electric force for the stretching of the polymer jet. This promotes a reduction in the diameter of the fibers[9,10]. Another important parameter to be controlled is the potential difference; high voltages allow the fluid to be transported quicker, allowing fibers with larger diameters to be obtained[11]. Furthermore, the variation of the distance between the injector and the collector also affects the morphology and diameter of the fibers. When working with a very large distance the electrospun fibers can break under their own weight, especially if they have small diameter. However, a minimum distance is required to allow the solution solvent to evaporate before reaching the collector plate, otherwise wet drops or fibers may form and, consequently, flattened or ribbon-like fibers may be obtained[11,12].
Encapsulation
The aim of encapsulation is to protect the active components from the adverse conditions of the medium such as high temperature, the presence of light and oxygen[13], pH change, presence of enzymes and other[14], by the application of a polymer coating. The encapsulating agents are classified in various categories as waxes, proteins, carbohydrates and food grade polymers such as Polyvinyl Alcohol (PVA)[15,16]. PVA is obtained by hydrolysis of polyvinyl acetate with fractions of molecular weights between 13,000 - 200,000 and whose physical properties, solution surface tension, rheological properties and water solubility depend on the degree of hydrolysis. PVA has the ability to form films and coatings that are highly resistant to traction, flexibility and have a low permeability to gases such as oxygen, carbon dioxide, and other vapors[17]. Toxicological studies have found that this substance has the lowest absorption rate in the body; its oral intake is approved with an Acceptable Daily Intake (ADI) dose of 50 mg / kg body weight[17,18]. Due to the chemical structure of the polyvinyl alcohol strong intermolecular interactions occur between the -OH groups and polar solvents.
Olive oil
Olive oil is composed of an oil fraction with 99% in the form of triglycerides and free fatty acids and presents a balance between omega-6 and omega-3 fatty acids. It is also a source of fat-soluble vitamins (A, D, E, K)[19,20]. It is possible to develop polyunsaturated oil electrospun fibers that allow their physical, oxidative and chemical stability given that the majority of the oil encapsulation Processes include micro and nanocapsules. However, little is known about the development and incorporation of fibers. Therefore, the objective of this work is to develop a process for the production of encapsulated olive oil fibers using the electrospinning method, with Polyvinyl Alcohol (PVA) as the coating material.
Materials and Methods
Encapsulation of Olive Oil in Emulsions of Polyvinyl Alcohol (PVA)
For the encapsulation tests, extra virgin olive oil Huasco (Chile) was used, obtained by cold extraction. To obtain microfibers and nanofibers, Polyvinyl Alcohol solutions (PVA) were prepared, with a molecular weight Mw 13000 - 23000 (Meyer, USA, Cat. 5425), and distilled water (Meyer, USA, Cat. 0345) The concentrations of the PVA solutions were 8, 10, 12, 14 and 16% w / w. The polyvinyl alcohol was dispersed in distilled water, with a propeller stirrer at 600 ± 20 rpm and 80 ± 1°C, for 30 minutes. Subsequently, when the temperature dropped to 25 ± 1°C, the oil was incorporated to form an emulsion, which was subjected to mechanical stirring using a 250 W commercial immersion mixer for 5 minutes until homogenized.
Rheological and physicochemical analysis of emulsions
The viscosity of the emulsions was evaluated at a temperature of 25 ± 1°C by a Rheolab QC Anton PaarRheometer (U.S.A.) using the DG24 concentric cylinder configuration and the Star Rheoplus Software[21]. The Conductivity was determined (mS) as was the hydrogen potential (pH) with Conductronic model PC18 (Mexico) at Room temperature.
Electrospinning process
The PVA solutions and oil emulsions were poured into the syringe plunger of the electro-syringe equipment Prendo (SEV, Mexico) and different voltage conditions were tested, ranging from 20 to 0 kV, based on the report by Zhang et al.[7] To obtain the fibers, the electrospinning equipment was operated under the following conditions: for the high voltage sources, 12 kV at the positive pole and 12 kV at the negative, an injection rate of 0.1 mL / h, a distance of 20 cm between the tip of the needle and the collector, rotational speed of the collector was 150 ± 5 rpm at a temperature of 241°C.
Morphological evaluation of fibers by SEM
Themorphological and microstructural evaluation of the obtained fibers, was determined by means of a low vacuum SEM microscope, model JSM-5300 (U.S.A.) with a potential difference of 10 kV and image amplifications of 5000, 10000 and 20000X. To determine the average fiber size, twenty measurements of the diameter were made using ImageJ[22].
Obtaining FTIR spectra of the fibers
The FTIR spectra permitted the identification of the principal characteristic spectral for each Components of the fibers. For this test, the VERTEX model FTIR 70 (U.S.A.) was employed.
Determination of the water content of the fibres
The water content of the fibers was determined by drying 1 - 2 g of sample material in a convection oven (Thermoline U.S.A) at 105°C for 24 h and then calculating the mass loss[23].
Determination of the water activity of the fibers
The water activity of the fibers was determined with an AQUA LAB (U.S.A.) hygrometer, calibrated previously, at a temperature of 25 ± 1°C.
Protective effect of PVA on the oxidation of olive oil
In order to evaluate the protection effect of the PVA encapsulating to olive oil, the films were irradiated in an ultraviolet light chamber, Prendo (SEV, Mexico), using a five lamp arrangement and light emission λ = 25 4 nm and exposure times of 30, 60 and 180 minutes. The L*, a* and b* color coordinates were evaluated on a Colorflex EZ45 colorimeter (Hunter Lab, USA). Subsequently, the color change of the fibers surface was determined using equation 1.
ΔE = √(L − L0)2 + (a − a0)2 + (b − b0)2 Equation
Statistical analysis
The results were analyzed by ANOVA, Tukey’s multiple comparison procedure, using the SPSS system for Windows (Version 15.0). All tests were carried out in triplicate; in the tables and figures the average values are reported. The differences were statistically accepted at p < 0.005.
Results and Discussion
The aqueous solutions of PVA presented values of electrical conductivity between 0.26 to 0.58 mS, which, accord-ing to the literature[24], can be successfully electrospun. The pH of the solutions was determined to lie between 6.46 and 6.98. From a statistical point of view, these differences were significant given that the pH is an indicator of the free ions present in the solution. PVA is a polymer that is characterized by having negatively charged residual monomers OH−, which impart a polar nature. The analysis of the obtained experimental data suggests an inverse relation between the concentration of the PVA and the conductivity of the polymer solution; the higher the concentration of PVA, the lower the electrical conductivity and higher the viscosity. With respect to the viscosity, this is an important parameter for the formation of the fibers. It represents the resistance of the sample to flow into the injector. Average values of viscosity of 0.28 to 0.90 Pa.s are reported here, which are suitable for the formation of fibers from the polymer, as reported by Sousa et al.[12] In (Table 1), the values of the viscosities, electrical conductivity and pH of the PVA solutions are shown.
Table 1: Values of the viscosity, electrical conductivity, pH of the PVA aqueous solutions and Their corresponding SEM micrographs for the fibers obtained.
1Media arithmetic of at least three replicates ± standard error. Values with the same letter within the columns are not significantly different according to the Tukey test (P > 0.05).
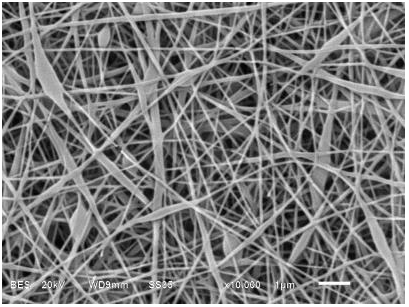
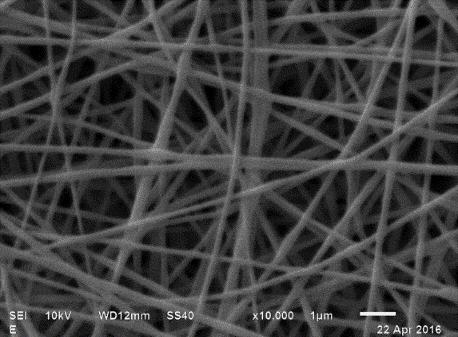
The Polyvinyl Alcohol (PVA) solutions at 8, 10 and 12% were electrospun and fiber formation was observed, whereas in PVA solutions at 14 and 16% no fibers were observed due to the high viscosity and low conductivity of the solutions. (Table 1) presents the images and the average diameters of the fibers obtained. The fibers at a concentration of 8% presented defects in their flattened morphologies which can be attributed to an excess in the moisture content in the formulation, i.e. During the process of electrospinning and transport of the fiber towards the collector the moisture content of the fiber did not evaporate out. This excess moisture content allows the fibers to deform on impact with the collector thereby forming a flattened shape. With respect to fibers at 10% concentration, it was possible to obtain homogeneous, non-deformed, cylindrical and regular fibers, indicating that the electrospinning conditions, as well as the PVA composition, were adequate. At 12% concentration, the experiments displayed aggregation defects due to the higher viscosity of the material and greater resistance of solution to flow during the passage through the injector. Such results were also reported by Fong et al[25]. The average size of the PVA fibers was determined from the micrographs; it is observed that the increase in the average diameter of the fibers is related to the concentration of PVA in the solution. The 10% PVA concentration was selected to carry out the encapsulation of the olive oil, given the homogeneous and well defined characteristics of the fibers.
Characterization of the emulsions
In order to identify the oil composition in the PVA solution to be electrospun, different oil compositions were tested. They were identified as follows: PVA + 4 (A) corresponding to a fiber composed of 10% polyvinyl alco-hol with an addition of olive oil 4% (w/w), PVA + 8 (B) fiber composed of 10% polyvinyl alcohol with an addition of 8% (w / w) olive oil and PVA + 12 (C) 10% polyvinyl alcohol fiber with an addition of 12% (w / w) olive oil. The emulsions had an average mouse; E-mail: llar diameter of 2.6 ± 0.3 μm. The physicochemical properties of the formulated emulsions are presented in (Table 2). The experimental results suggest an inverse relationship between the oil concentration and the conductivity and viscosity of the emulsion. The values of the conductivity of the emulsions varied according to the concentration of olive oil added. Although, as stated about, PVA is a polymer characterized by having negatively charged residual monomers reflected by the conductivity of the sample, during the addition of the olive oil, the conductivity was significantly reduced by the hydrophobic nature of the oil; the hydrophilic sites were diminished or masked. This phenomenon reduced the feasibility of creating nanofibers. Furthermore, there was an increase in the viscosity of the emulsion, which difficult the flow into the injector limiting the possibility of electrospinning. The same conditions for PVA fibers were also used for the electrospun emulsions. The micrographs of the fibers obtained are presented in (Table 2); in them it can be observed that the fibers that encapsulated the oil presented slight bumps. The composition of the PVA 10% + 4 and PVA 10% + 8 emulsions were suitable for obtain regular fibers, presenting a linear morphology with bulges due to the presence of olive oil. The measurement of diameter of fibers of PVA 10% + 4 showed that it was possible to obtain nanofibers, while PVA 10% + 8, only microfibers could be produced. The PVA 10% + 12 fibers had a non-uniform linear morphology, which implies that a high concentration of oil limits the possibility of fiber formation.
Table 2: Values of the viscosity, electrical conductivity and pH of the PVA encapsulated oil Aqueous solutions and their corresponding SEM micrographs for the fibers obtained.
1Media arithmetic of at least three replicates ± standard error. Values with the same letter within the columns Are not significantly different according to the Tukey test (P > 0.05).
Validation of the olive oil encapsulation in the fibers by FTIR
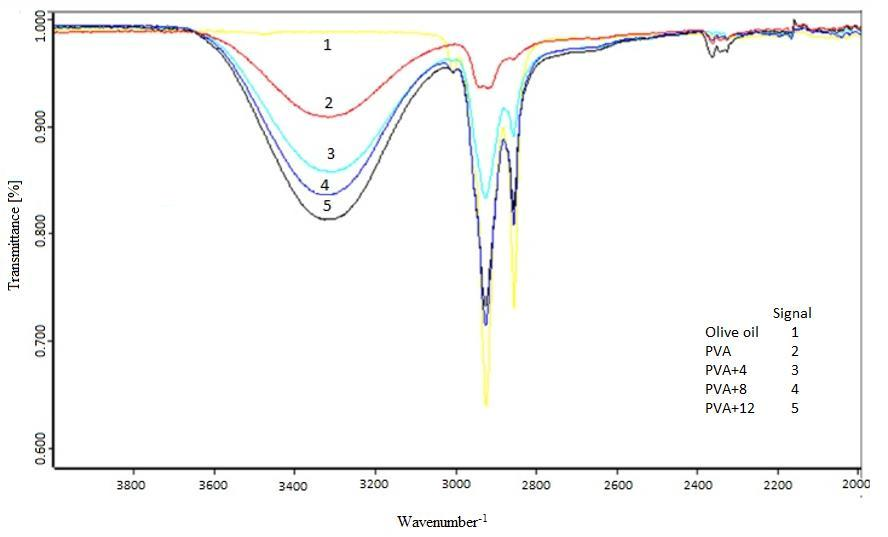
The symmetrical and asymmetric extension vibrations of the H-O-H bonds are located in the range 3490 - 3280 cm-1 and are associated with the water containing the fibers of PVA and oil. The band centered at 3007 cm-1 is assigned to the symmetrical extension vibrations of the linear chains =C-H of the alkenes present in the oil. This same vibration is observed in olive oil. On the other hand, the bands centered on 2957, 2922, 2873 and 2852 cm-1 are assigned to the asymmetric and symmetrical extension vibrations of C-H in lipids[26,27]. With respect to the FTIR of PVA, a wide band belonging to the alkyl group (ν = 2850 - 3000 cm-1), hydroxyl bands typical of alcohol –OH (OH-OH stretching band at ν = 3600 - 3650 cm-1), and hydrogen bonded band (v = 3200 - 3570 cm-1), due to the intramolecular and intermolecular hydrogen bonds that develop between the PVA chains[28] are observed. It is important to note that the analysis of the FTIR spectra made it possible to identify the olive oil encapsulated in the fibers and it was evident there was no interaction between the oil and PVA figure 2.
Figure 2: shows the FTIR spectra of PVA and PVA fibers with encapsulated oil and liquid oil.
Water activity and moisture content
The values obtained for the water activity (aw) of the fibers were PVA, 0.386 ± 0.002; PVA + 4, 0.378 ± 0.002; PVA + 8, 0.385 ± 0.002; PVA + 12, 0.407 ± 0.002. These values are very low which implies a low microbial growth in the fibers due to its low moisture content and low water activity during its storage. This behavior is reflected by the low moisture content of the oil fibers 0.104 ± 0.001; PVA + 4, 0.128 ± 0.002; PVA + 8, 0.144 ± 0.002; PVA + 12, 0.167 ± 0.002 kg H2O / kg dry solid.
Protective effect of PVA on the oxidation of olive oil
Irradiating the fibers with UV radiation indicated how photostable the fibers are. For exposure times of 30 and 60 minutes, no significant difference to the color of the fibers was observed. However, a significant change in the color of the surface of the fiber with exposure of 180 minutes was evident, turning yellowish color. The ΔE results are presented in (Table 3).
Table 3: Mean experimental values of the color change of UV irradiated fiber webs.
| Sample | ΔE | ||
|---|---|---|---|
| 30 min | 60 min | 180 min | |
| PVA | 0.10 ± 0.02a1 | 0.21± 0.07a2 | 0.92 ± 0.08a3 |
| PVA+4 | 0.22 ± 0.06b1 | 0.41 ± 0.08b2 | 1.61± 0.25b3 |
| PVA+8 | 0.32 ± 0.08c1 | 0.50 ± 0.09c2 | 4.10 ± 0.45c3 |
| PVA+12 | 0.31± 0.07c1 | 0.61± 0.10d2 | 5.13 ± 0.58d3 |
1Media arithmetic of at least three replicates ± standard error. Values with the same initial lowercase letter within the columns are not significantly different according to the Tukey test (P > 0.05). Numeric values with the same superscript within the lines is not significantly different according to the Tukey test (P > 0.05).
The color difference (ΔE) was higher for an exposure time of 180 min in the four exposed fiber samples, being higher for PVA + 12 due to a higher concentration of encapsulated olive oil (Table 3). From these it can be concluded that the encapsulation process was efficient using a 60 minute exposure time. Regarding the values of L, a and b, it was observed that the changes in the parameters L and b were greater in magnitude. This means that, during their exposure to the UV radiation, the fibers presented an increase in the yellow tonality (b), a result of the initiation of oxidation reactions of the olive oil encapsulated in the fibers.
Conclusion
The electrospinning technique allowed the formation of PVA microfibers in which olive oil was encapsulated. The possibility of forming PVA nanofibers was identified, after which it was found that the morphology of the nano-fibers varied as a function of the concentration of PVA in the solution, presenting fibers with varying diameter of values between 0.1206 and 0.3599 μm. The electrospinning technique permitted the encapsulation of olive oil in PVA microfibers, with an average diameter of PVA + 4, 0.2038 ± 0.0280 μm; PVA + 8, 0.4202 ± 0. 0100 μm and PVA + 12, 0.7041 ± 0.0350 μm. The encapsulation of olive oil was verified by FTIR spectroscopy, highlighting the potential of the electrospinning technique through the identification of the double bonds corresponding to the unsaturation of olive oil. It was determined that the fibers obtained had low water content since 99% of the solvent was evaporated during electrospinning.
Acknowledge:
The first author acknowledges the support of the Consejo Nacional de Ciencia y Tecnologia (CONACYT) through a scholarship to pursue a Master in Chemical Engineering within the master program of the Benemérita Universidad Autónoma de Puebla.
Conflict of interest:
The authors declare that there is no conflict of interest.
References
- 1. Bhardwaj, N., Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. (2010) Biotechnol Adv 28(3): 325-347.
Pubmed || Crossref || Others - 2. Mendes, A.C., Stephansen, K., Chronakis, I. Electrospinning of Food Proteins and Polysaccharides. (2017) Food Hydrocoll 68: 53-68.
Pubmed || Crossref || Others - 3. Mercante, L.A., Scagion, V.P., Migliorini, F.L., et al. Electrospinning-Based (Bio)sensors for Food and Agricultural Applications: A review. (2017) Trends Analyt Chem 91: 91-103.
Pubmed || Crossref || Others - 4. Lee, K.H., Kim, H.Y. Khil, M.S., et al. Characterization of Nano–Structured Poly (E– Caprolactone) Nonwoven Mats Via Electrospinning. (2003) Polymer 44(4): 1287-1294.
Pubmed || Crossref || Others - 5. Repanas, A., Andriopoulou, S., Glasmacher, B. The Significance of Electrospinning as a Method to Create Fibrous Scaffolds for Biomedical Engineering and Drug Delivery Applications. (2016) J Drug Deliv Sci Technol 31: 137-146.
Pubmed || Crossref || Others - 6. Tylingo, R., Gorczyca, G., Mania, S., et al. Preparation and Characterization of Porous Scaffolds from Chitosan-Collagen-Gelatin Composite. (2016) React Funct Polym 103: 131-140.
Pubmed || Crossref || Others - 7. Zhang, Z., Wu, Y., Wang, Z., et al. Fabrication of Silver Nanoparticles Embedded into Polyvinyl Alcohol (Ag/PVA) Composite Nanofibrous Films through Electrospinning for Antibacterial and Surface-Enhanced Raman Scatterign (SERS) Activites. (2016) Mater Sci Eng C Mater Biol Appl (69): 462-469.
Pubmed || Crossref || Others - 8. Doshi, J., Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. (1995) J Electrostat 35(2-3): 151-160.
Pubmed || Crossref || Others - 9. Sencadas, V., Correia, D.M., Areias, A., et al. Determination of the Parameters Affecting Electrospun Chitosan Fiber Size Distribution and Morphology. (2012) Carbohydr Polym 87(2): 1295-1301.
Pubmed || Crossref || Others - 10. Ohkawa, K., Kim, H., Lee, K. Biodegradation of Electrospun Poly-(?-caprolactone) Non-woven Fabrics by Pure-Cultured Soil Filamentous Fungi. (2004) J Polym Environ 12(4): 211-218.
Pubmed || Crossref || Others - 11. Yan, S., Li, X., Dai, J., et al. Electrospinning of PVA/Sericin Nanofiber and the Effect on Epithelial-Mesenchymal Transition of A549 Cell. (2017) Mater Sci Eng C Mater Biol Appl 79(1): 436-444.
Pubmed || Crossref || Others - 12. Sousa, A.M.M., Souza, H.K.S., Uknalis, J. et al. Electrospinning of Agar/PVA Aqueous Solutions and its Relation with Rheological Properties. (2015) CarbohydrPolym 115: 348-355.
Pubmed || Crossref || Others - 13. Nedovic, V., Kalusevic, A., Manojlovic, V., et al. An overview of Encapsulation Technologies for Food Applications. (2011) Procedia Food Sci 1: 1806-1815.
Pubmed || Crossref || Others - 14. Mozafari, R.M., Flanagan, F., Matia-Merino, L., et al. Recent Trends in the Lipid Based Nanoencapsulation of Antioxidants and their Role in Foods. (2006) J Sci Food Agric 86(13): 2038-2045.
Pubmed || Crossref || Others - 15. Fuchs, M., Turchiuli, C., Bohin, M., et al. Encapsulation of Oil in Powder Using Spray Drying and Fluidized Bed Agglomeration. (2006) J Food Eng 75(1): 27-35.
Pubmed || Crossref || Others - 16. Madene, A., Jacquot, M., Scher, J., et al. Flavour Encapsulation and Controlled Release – a Review. (2006) Int J Food Sci Technol 41(1): 1-21.
Pubmed || Crossref || Others - 17. Tsai, R.Y., Kuo, T.Y., Hung, S.C., et al. Use of Gum Arabic to Improve the Fabrication of Chitosan-Gelatin-based Nanofibers for Tissue Engineering. (2015) Carbohydr Polym 115: 525-532.
Pubmed || Crossref || Others - 18. DeMerlis, C.C., Schoneker, D.R. Review of the Oral Toxicity of Polyvinyl Alcohol (PVA). (2003) Food Chem Toxicol 41(3): 319-326.
Pubmed || Crossref || Others - 19. Roselli, L., Clodoveo, M.L., Corbo, F., et al. Are Health Claims a Useful Tool to Segment the Category of Extra-virgin Olive Oil? Threats and Opportunities for the Italian Olive Oil Supply Chain. (2017) Trends Food Sci Technol 68: 176-181.
Pubmed || Crossref || Others - 20. Kanavouras, A., Coutelieris, F.A. Systematic Transition from Description to Prediction for the Oxidation in Packaged Olive Oil. (2017) Food Chem 229: 820-827.
Pubmed || Crossref || Others - 21. Okutan, N., Terzi, P., Altay, F. Affecting Parameters on Electrospinning Process and Characterization of Electrospun Gelatin Nanofibers. (2014) Food Hydrocoll 39: 19-26.
Pubmed || Crossref || Others - 22. https://imagej.nih.gov/ij/
Pubmed || Crossref || Others - 23. AOAC International. Official Methods of Analysis 16th ed Virginia USA. (1995) AOAC International.
Pubmed || Crossref || Others - 24. Ohshima, H., Kondo, T. pH Dependence of Electrostatic Interaction between Ion-penetrable Membranes: Electrostatic Interaction; Membrane Interaction. (1988) Biophys Chem 32(2-3): 161-166.
Pubmed || Crossref || Others - 25. Fong, H., Chun, I., Reneker, D.H. Beaded Nanofibers Formed During Electrospinning. (1999) Polymer 40(16): 4585-4592.
Pubmed || Crossref || Others - 26. Mishra, S.K., Kannan, S. Development, Mechanical Evaluation and Surface Characteristics of Chitosan/Polyvinyl Alcohol based Polymer Composite Coatings on Titanium Metal. (2014) J Mech Behav Biomed Mater 40: 314-324.
Pubmed || Crossref || Others - 27. Shriner, R.L., Hermann, C.K.F., Morrill, T.C., et al. The Systematic Identification of Organic Compounds, 8th edition New Jersey USA. (2003) Wiley 736.
Pubmed || Crossref || Others - 28. Fonseca dos Reis, E., Campos, F.S., Pereira Lage, A., et al. Synthesis and Characterization of Poly (Vinyl Alcohol) Hydrogels and Hybrids for rMPB70 Protein Adsorption. (2006) Materials Research 9(2).
Pubmed || Crossref || Others