Evaluation of a Consciousness Energy Healing Based Test Formulation for Skin Health
Dahryn Trivedi1, Snehasis Jana2*
Affiliation
1Trivedi Global, Inc. Henderson, Nevada, USA
2Trivedi Science Research Laboratory Pvt. Ltd, Bhopal, Madhya Pradesh, India
Corresponding Author
Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd, Bhopal, Madhya Pradesh, India, Email: publication@trivedieffect.com
Citation
Snehasis, J., et al. Evaluation of A Consciousness Energy Healing Based Test Formulation for Skin Health (2019) Invest Demerol and Venereol Res 5(1): 7-13.
Copy rights
© 2019 Snehasis, J., This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Consciousness energy healing; The Trivedi Effect®; Extra cellular matrix; BrdU assay; Wound healing; Skin protection
Abstract
This study was investigated the effect of Consciousness Energy Healing (The Trivedi Effect®) based test formulation and DMEM medium on skin health in B16-F10, HaCaT, and HFF-1 cells. The test formulation and DMEM were assigned into two parts. First part was received the Biofield Treatment by a renowned Biofield Energy Healer, Dahryn Trivedi and were defined as the Biofield Treated samples (BT), while the second parts were denoted as the untreated test samples (UT). MTT assay data showed more than 76 % cells were viable in all the tested concentrations in three different cell lines, indicating that the test formulation was safe and nontoxic. The BrdU assay showed the percent cell proliferation was increased by 15.88 % in the UT-DMEM + BT- Test formulation compared to the UT-DMEM + UT- Test formulation group. Elastin was increased significantly (p ≤ 0.001) by 64.37 %, 29.46 % and 38.75 % in UT-DMEM + BT-Test formulation, BT-DMEM + UT-Test formulation, and BT-DMEM + BT-Test formulation, respectively at 100 µg / mL compared to the untreated test formulation. Hyaluronic acid was significantly increased by 17.74 %, 96.20 %, and 25.03% in UT-DMEM + BT- Test formulation, BT-DMEM + UT-Test formulation, and BT-DMEM + BT-Test formulation groups, respectively at 10 µg / mL compared to untreated group. The level of melanin was significantly decreased by 8.60% (p ≤ 0.05), 12.68 % (p ≤ 0.001), and 21.73% (p ≤ 0.001) in the UT-DMEM + BT- Test formulation group at 0.1, 1.0, and 10 µg / mL, respectively compared to the untreated group. Protection of skin cells after UV-B exposure data showed that the cell viability was significantly increased by 31.15 % and 10.84 % in the UT-DMEM + BT-Test formulation and BT-DMEM + UT-Test formulation groups, respectively at 50 µg / mL compared to the untreated group. Wound healing data exhibited that the wound area was recovered with new cells by 84.91 %, 92.31 %, 35.09 %, and 41.67 % at 10, 50, 100, and 200 µg / mL in the BT-DMEM + BT- Test formulation group, respectively in Ha Ca T cells compared to the untreated group. The experimental data envisaged that the Biofield Energy Treated DMEM and test formulation showed significant skin protective activity. Thus, the Biofield Energy Treated test formulation could be useful for the development of effective cosmetic products for the nourishment of several skin problems such as fine lines, wrinkles, texture, pore size, elasticity, skin color / clarity, redness, hydration, to maintain the overall skin quality, etc.
Introduction
Healthy and well-functioning skin act as an important barrier to protect against dehydration, invading microorganisms, allergens, irritants, Reactive Oxygen Species (ROS) and radiation. The skin barrier specifically adjusted to allow penetration. Hence regular skin care may increase the capacity of skin regeneration, elasticity, and smoothness[1,2]. However, it is possible to reduce the process of degeneration and formation of wrinkles by changing the structural constituents like collagen, elastin, hyaluronic acid etc. At present, no such technology is available directly to alter the structural components of skins and thus minimize skin degeneration or indirectly to promote skin regeneration. Although some products are available in the market to promote the natural synthesis of the basic constituents of skin[3-5]. Besides, the skin wrinkles can be prevent by reducing the inflammation by providing some antioxidants[6]. In this aspect authors have formulated a proprietary formulation based on the essential minerals (zinc chloride, ferrous sulphate, copper chloride, and magnesium gluconate) and vitamins (pyridoxine hydrochloride cyanocobalamin, and cholecalciferol). Every ingredient has a potential skin health benefits in the form of various formulations, as well as cosmeceuticals. Among these minerals, zinc is used as an essential cofactor of various metallo enzymes and it protects the skin from UV radiation and reduce inflammation. Abnormal metabolism and deficiency of zinc can causes hereditary disorders, acrodermatitis enteropathica[7-9]. Skin aging can aggravates due to environmental pollutants and exposure to UV radiation. Ascorbic acid (Vitamin C) act as a powerful antioxidant that can help to rejuvenate aged and photodamaged skin and also used for the production of collagen[10,11].
Most of US citizens are regularly using some form of CAM[12]. in their day-to-day life. Researchers reported that a short-lived electrical action potential exists in the mammalian cells such as muscles, neurons, and the endocrine system. The cells present in the Central Nervous System CNS) can communicate with each other with the help of an electrical signals that propagate the nerve impulses[13]. Biofield can present around the human body which was found by various techniques such as electrocardiography, electroencephalogram, and electromyography[14]. Thus, a Biofield Healing Practitioner has the significant ability to harness the energy from the environment and can transmit it into any objects. The object (s) always receives the energy and responds in a useful way that is called “Biofield Energy Treatment”. This process is known as “Biofield Energy Healing Treatment”. Biofield Energy Healing has been approved as an alternative method that has an impact on various properties of living organisms in a cost-effective manner[15,16]. The Trivedi Effect®- unique Biofield Energy Treatment has been known to alter the response in a wide-spectrum field in living organisms and non-living systems viz. skin health[17-20]. Materials science[21-23] Agriculture[24,25]. Microbiology[26-28]. Biotechnology[29,30]. Based on the excellent outcome of the Biofield Energy Treatment, the authors designed this study to investigate the impact of the Biofield Energy Healing based test formulation and DMEM on various skin health parameters using three cell lines such as human foreskin fibroblast (HFF-1), human keratinocytes (HaCaT), and mouse melanoma (B16-F10) cells.
Materials and Methods
Chemicals and Others Requirement: Magnesium (II) gluconate hydrate, zinc chloride, cyanocobalamin (vitamin B12), and pyridoxine hydrochloride (vitamin B6) were purchased from TCI, Japan. Cholecalciferol (vitamin D3), iron (II) sulphate, copper chloride, kojic acid, and L-ascorbic acid were purchased from Sigma-Aldrich, USA. ELISA kits were obtained from CUSABIO and CusAb Co Pvt. Ltd., USA. Epidermal growth factor: (EGF), Fetal bovine serum: (FBS), and Dulbecco’s Modified Eagle’s Medium: (DMEM) were procured from Gibco, ThermoFisher, USA. Antibiotics solution (penicillin-streptomycin) was procured from HiMedia, India, while 3-(4, 5-dimethyl-2-thiazolyl) (2, 5-diphenyl-2H-tetrazolium) (MTT), Direct Red 80 and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma, USA. All the other chemicals used in this experiment were analytical grade procured from India.
Cell Culture: The human foreskin fibroblast-1 (HFF-1) cells were obtained from American Type Culture Collection (ATCC), USA. B16-F10 (mouse melanoma) cells were obtained from National Centre for Cell Science (NCCS) Pune. Both the cells were maintained as per Nayak et al. 2018[31].
Study Regimen: The experimental groups consisted of cells in normal control (G1), vehicle control (G2) group (0.05% DMSO), positive control (G3) group and test groups. The test groups included the combination of Biofield Energy Treated and untreated test formulation / DMEM. It consisted of four major treatment groups on specified cells with UT-DMEM + UT- Test formulation (G4), UT-DMEM + BT- Test formulation (G5), BT-DMEM + UT- Test formulation (G6), and BT- DMEM + BT- Test formulation (G7).
Consciousness Energy Healing Treatment Strategies: The test formulation and DMEM were divided into two parts. One of each part of the test formulation and one part of the DMEM were considered as untreated samples, and were subjected to “sham” healer under the same laboratory conditions. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. While the other parts of each were defined as the Biofield Treated samples. Both the samples were kept under standard laboratory conditions at the research laboratory of Dabur Research Foundation, New Delhi, India. The treated group samples were subjected to the Consciousness Energy Healing (The Trivedi Effect®) Treatment by a renowned Biofield Energy Healer, Dahryn Trivedi remotely for ~5 minutes from U.S.A. The Biofield Energy Healer, Dahryn Trivedi, never visited the laboratory in person, nor had any contact with the test samples. After that, the Biofield Energy Treated and untreated samples were kept in similar sealed conditions and used for this experiment.
Evaluation of Cell Viability by MTT Assay: The cell viability was done using MTT assay in three cell lines viz. B16-F10, HFF-1, and Ha Ca T. The details stepwise protocol was followed as per Nayak et al. 2018 with some modifications[31]. The concentration exhibiting % cytotoxicity of less than 30% was considered as non cytotoxic[32].
Cell Proliferation by 5-bromo-2'-deoxyuridine (BrdU) Assay: HFF-1 cells were used for the assessment of cell proliferation. Briefly, the procedure of this technique was followed by manufacturer’s instructions with some modifications as per Nayak et al. 2018[31].
Evaluation of Collagen, Elastin, and Hyaluronic Acid: Synthesis of extracellular matrices components (i.e. elastin, collagen, and hyaluronic acid) in HFF-1 cells was performed by manufacturer’s instructions[33], with some modifications as per Nayak et al. 2018[31].
Determination of Melanin: B16-F10 cells were used for melanin synthesis estimation. Rest over details steps were considered by manufacturer’s instructions[34]. With some modifications as per Nayak et al. 2018[31].
Anti-wrinkle Activity: UV-B induced stress was evaluated in HFF-1 cells and the cell viability was estimated in the presence of the test samples. The whole process was maintained as per manufacturer’s instructions[35]. with some modifications as per Nayak et al. 2018[31].
Scratch Assay for Wound Healing Activity: HFF-1 and Ha Ca T cells were used for the evaluation of wound healing activity and the details procedure was followed as per manufacturer’s instructions[36]. With some modifications by Nayak et al. 2018[31].
Statistical Analysis: All the experimental data were assessed using Sigma-plot (V11.0). Each experiment was carried out in triplicates and represented as mean ± standard error of mean: (SEM). For multiple group comparison one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dennett’s test. For comparison between two groups Student’s t-test was used. Statistically significant values were set at the level of p ≤ 0.05.
Results and Discussion
Evaluation of Cell Viability by MTT Assay: For the evaluation of the viable cells three different cell lines (HFF-1 Ha Ca T, and B16-F10) were used in this experiment. MTT assay was used and the percent cell viability data are shown in Figure 1A to 1C. The result of cell viability in the HFF-1 and HaCa T cells exhibited > 80% and >73% viable cells, respectively in the tested concentrations ranges from 1 to 200 µg / mL (Figure 1A and 1B). The selected concentrations were used for the estimation of extracellular matrix (ECM) synthesis like collagen, elastin, and hyaluronic acid and wound healing activity by scratch assay in HFF-1 cells and wound healing activity in Ha Ca T cells. The percentage of viable cells in the B16-F10 cells data exhibited that the test groups were non-cytotoxic (i.e. percentage cell viability value > 101%). The tested concentrations were used further for the measurement of melanin level from 1 to 100 µg / mL (Figure 1C).

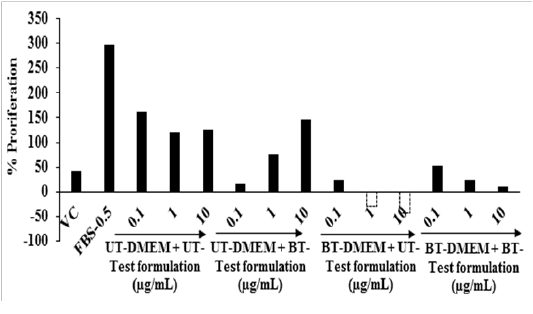
Figure 1: Effect of the test formulation on cell viability in different cells at various concentrations A. HFF-1 cells after 72 hours of treatment. B. HaCaT cells after 24 hours of treatment. C. B16-F10 cells after 24 hours of treatment. LA: L-Ascorbic acid; EGF: Epidermal growth factor; KA: Kojic acid
Cell Proliferation by 5-bromo-2'-deoxyuridine (BrdU) Assay: The cell proliferation was analyzed with the help of BrdU assay and the data are shown in Figure 2. The percentage of cell proliferation was increased by 626.83% in the G3 group (FBS-0.5 µg / mL) compared to the G2 group (vehicle control, VC). A result of the percent cell proliferation was increased by 15.88% in the G5 groupat 10 µg / mL compared to the G4 group. Cell proliferation is vital for cellular homoeostasis and maintenance of an organism. The bromodeoxyuridine (BrdU) assay was used for the evaluation of the rate of DNA replication, analysis of metabolic activity and recognitions of cell surface antigen activity[37]. Overall, the Biofield Energy Treated test formulation showed significant cell proliferation response compared to the untreated group, due to the Biofield Energy Healing Treatment.
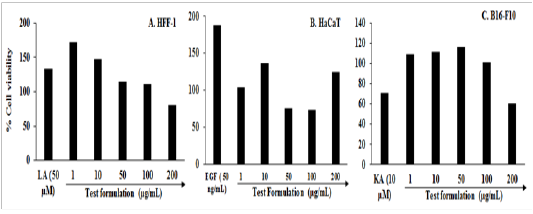

Figure 2: Effect of the test formulation on cellular proliferation after 48 hours using BrdU assay. VC: Vehicle control; FBS: Fetal bovine serum (µg / mL); UT: Untreated; BT: Biofield Treated
Evaluation of Collagen, Elastin, and Hyaluronic Acid
Collagen: The expression of collagen in the presence of the test formulation and DME Min HFF-1 cell is presented in Figure 3. The level of collagen in the G2 was 122.29 ± 4.46 µg / mL and it was significantly (p ≤ 0.001) increased by 29.20% in the G3 (158 ± 0.00 µg / mL). The level of collagen did not show any significant alteration in all the treated groups compared to the G4. Several stimuli causes chronic wounds, in which there was an elevation of matrix metallo proteinases (MMPs) enzymes that degraded the both viable as well as non-viable collagen[38]. Collagen is an important component responsible for wound healing[39]. Overall due to damage of collagen the repair process also hampered. Therefore, to control collagen metabolism might be useful for a variety of therapeutic and cosmetic applications. Altogether in this experiment, the level of collagen synthesis was altered to some extent in the Biofield Energy Treated test formulation and DMEM group.

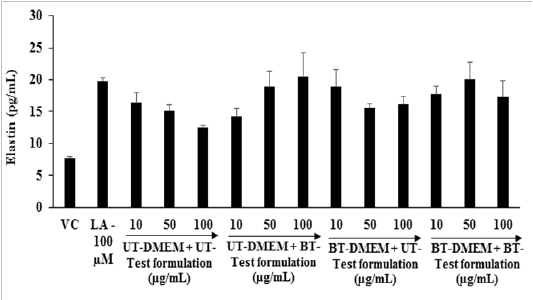
Figure 3: Effect of the test formulation and DMEM on the expression of collagen in human foreskin fibroblast cells (HFF-1). VC: Vehicle control; LA: L-Ascorbic acid. ***p ≤ 0.001 vs VC (G2) using Student’s t-test.
Elastic: The effects of the test formulation and DMEM on the elastic level in the human foreskin fibroblast cells (HFF-1) are presented in Figure 4. The level of elastin was found as 7.69 ± 0.29 and 19.81 ± 0. 41 pg / mL in the G2 and G3, respectively. The level of elastin was significantly increased by 15.60% and 8% at 10 µg / mL in the G6 and G7 groups, respectively compared to the G4. Moreover, at 50 µg / mL the level of elastin was significantly (p ≤ 0.001) elevated by 25.71% and 32.87% in G5 and G7 groups, respectively compared to G4. Further, at 100 µg / mL the expression of elastin was also significantly (p ≤ 0.001) increased by 64.37%, 29.46% and 38.75% in the G5, G6, and G7 groups, respectively compared to G4. Elastin is required to maintain the mechanical, recoil and resistance properties to the skin and to impart a wide-range of cellular activities like cell migration and proliferation, matrix synthesis, and protease production[40]. Based on the internal properties of elastin, its play a vital role in wound healing process. The intrinsic aged skin is due to dryness and lack of elastin as found in youthful skin[41]. Overall, the level of elastin synthesis was improved significantly in the Biofield Energy Treated test formulation and DMEM, due to The Trivedi Effect® - Consciousness Energy Healing.
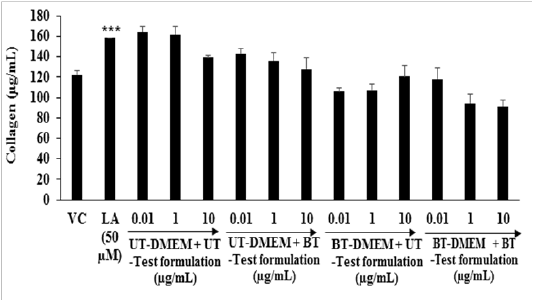

Figure 4: Effect of the test formulation and DMEM on the elastin level in human foreskin fibroblast cells (HFF-1).
Hyaluronic acid: The effects of the test formulation with DMEM on HA level in human foreskin fibroblast cells (HFF-1) are represented in Figure 5. The synthesis of HA in the presence of ascorbic acid (100 µM), showed significantly increased by 11.21% compared with the G2 group (26.14 ± 0.24 ng / mL). The level of HA was significantly (p ≤ 0.001) increased by 17.74%, 96.20%, and 25.03% in the G5, G6, and G7 groups, respectively at 10 µg / mL compared to G4. Further, at 50 µg / mL the HA level was significantly (p ≤ 0.001) increased by 22.41% and 34.51% in G6 and G7 groups, respectively compared to G4. Additionally, the level of HA was significantly (p ≤ 0.001) increased by 22.88% and 38.00% in G6 and G7 groups with respect to G4 at 100 µg / mL. The overall data suggested that the Biofield Energy based test formulation and DMEM have the significant capacity to increase the extra-cellular skin component, hyaluronic acid.

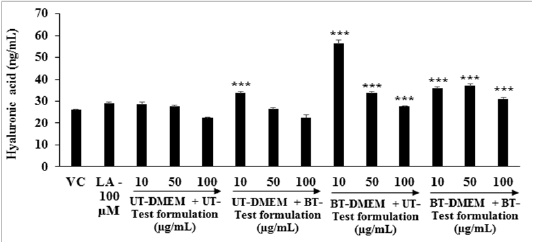
Figure 5: Effect of the test formulation on hyaluronic acid (HA) in human foreskin fibroblast cells (HFF-1). ***p≤0.001 vs G4.
Determination of Melanin
The effect of the test formulation on alpha-MSH stimulated melanin synthesis in B16-F10 cells is shown in Figure 6. The level of melanin in the alpha-MSH group was 14.08 ± 0.08 µg / mL and it was significantly (p ≤ 0.001) reduced by 74.57% in the kojic acid (KA) group (3.58 ± 0.17 µg / mL) at 10 mM. The cellular content of melanin was significantly reduced by 8.60% (p ≤ 0.05), 12.68% (p ≤ 0.001), and 21.73% (p ≤ 0.001) in G5 at 0.1, 1.0, and 10 µg / mL, respectively compared to G2. The rest of the treatment groups showed an alteration of melanin concentration with respect to G2. Thus,it can be concluded that the Biofield Energy Treated test formulation significantly inhibited the melanin production in the B16-F10 cells. This improvement might be beneficial for the development of cosmeceutical products for hyper pigmentation and different types of skin conditions.

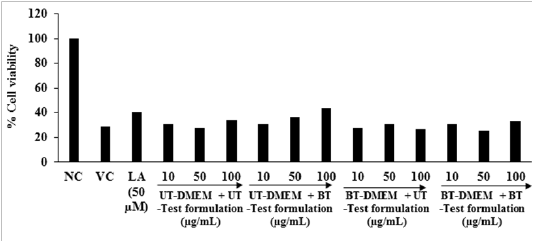
Figure 6: Effect of the test formulation and DMEM on alpha-MSH stimulated melanin synthesis in B16-F10 cells. KA: Kojic acid (mM); α-MSH: Alpha-melanocyte-stimulating hormone.* p≤ 0.05 and ***p ≤ 0.001 vs G4, ###p ≤ 0.001 vs α-MSH.
Anti-wrinkle Activity: The effects of the test formulation and DMEM against UV-B challenges are shown in Figure 7. The cell viability was measured using hemocytometer. The cells were subjected to lethal dose of UV-B irradiation (200 mJ / cm2) and found 24.41% cell viability. Cell viability in the normal control (NC) group (G1), G2, and G3 (L-ascorbic acid) groups was 100%, 28.63%, and 40.4%, respectively. After UV-B induced stress condition the level of the percent cell viability was significantly increased by 31.15% and 10.84% in G5 and G6 groups, respectively at 50 µg / mL compared to G4. Furthermore, the percent cell viability was significantly increased by 30.85% in G5 at 100 µg / mL compared to G4. The rest of the treatment groups exhibited an alteration with respect to G4. Numerous factors are responsible for skin wrinkles viz. environmental factors (ultraviolet radiation and smoking), aging, deficiency of estrogen, and due to genetic alteration[42,43]. Among them, aging is one of the primary factors responsible for skin wrinkles. In humans, due to aging the skin becomes thin and level of elasticity, collagen, etc. were decreased[44,45]. The study data suggests that the Biofield Energy Treated test formulation and DMEM significantly improved the anti-wrinkle activity, which should be used for the preparation of anti-wrinkling formulation.

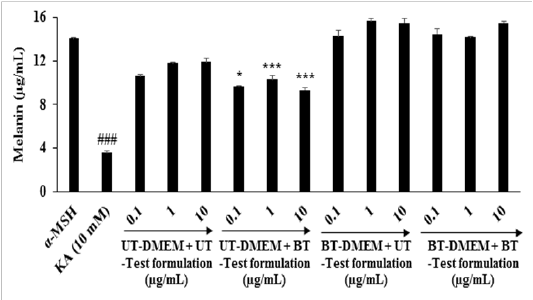
Figure 7: Anti-wrinkle activity in terms of percentage restoration of the cell viability in HFF-1 cells after 20 hours pretreatment of the test formulation and DMEM before UV-B challenge. NC: Normal control
Scratch Assay for Wound Healing Activity: Wound healing activity was performed using in vitro scratch assay and the representative photomicrographs are presented in Figure 8. The cell coverage area was increased by 35%, 43%, and 20% in G5, G6, and G7 groups, respectively at 1 µg / mL in Ha Ca T cells compared to G4. The cell coverage area was increased by 38% and 54% in G6 and G7 groups, respectively at 10 µg / mL compared to G4 in Ha Ca T cells. Moreover, at 50 µg / mL the cell coverage area was increased by 17%, 12%, and 31% in G5, G6, and G7 groups, respectively in Ha Ca T cells compared to G4 (Figure 8A). On the others hand, the cell coverage area was increased by 10% and 15% in G6 and G7 groups, respectively at 200 µg / mLcompared to G4. Further, the maximum percentage of cell migration was 78.9%, 45.1%, 52.3%, and 50.4% in G4, G5, G6, and G7 groups, respectively in HFF-1 cells compared to the baseline control (Figure 8B). The scratch assay is a well-established technique for the evaluation of wound healing activity that measured the cell-matrix, cell-to-cell interactions, and cell migration during the wound healing process[40]. The findings showed excellent wound closure activity in Ha Ca T cells compared to the untreated.

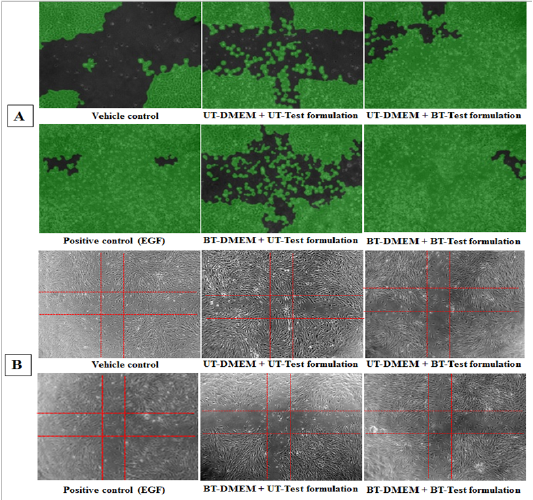
Figure 8: Effect of the test items on wound healing activity after 16 hours of treatment. Specimen photomicrographs (X10) of wound closure and cell migration are shown in A. HaCaT cells and B. HFF-1.
Conclusion
The cell viability exhibited more than 76% cells were viable in all the tested concentrations and found as nontoxic. The cell proliferation (%) using BrdU assay was increased by 15.88% in the UT-DMEM + BT-Test formulation compared to the UT-DMEM + UT-Test formulation group.Elastin was significantly increased by 38.75%, 64.37%, and 29.46% in the (BT-DMEM + BT-Test formulation), (UT-DMEM + BT-Test formulation), and (BT-DMEM + UT-Test formulation) groups, respectively at 100 µg / mL than untreated. Hyaluronic acid was significantly increased by 25.03%, 17.74%, and 96.20% in the BT-DMEM + BT-Test formulation, UT-DMEM + BT-Test formulation, and BT-DMEM + UT-Test formulation groups, respectively at 10 µg / mL compared to the untreated group. Melanin level was significantly reduced by 12.68% and 21.73% in the UT-DMEM + BT-Test formulation group at 1 and 10 µg / mL, respectively with respect to the untreated group. Cellular protection with respect to UV-B, revealed that the level of cell viability was increased by 31.15% and 10.84% in the UT- DMEM + BT- Test formulation and BT-DMEM + UT-Test formulation groups at 50 µg / mL, respectively compared to the untreated group. Wound healing data showed significant wound closure and cell migration in all the tested groups in Ha CaT cells compared to the untreated. Overall, the Consciousness Energy Healing (The Trivedi Effect®) Treated test formulation and DMEM have shown significant skin protective effects on various skin health parameters such as wrinkling, aging, skin whitening, and wound healing. Thus, the Biofield Treated test formulation would be suitable for the management of wounds and various skin-related disorders viz cellulitis, skin abscess, pimples, scabies, impetigo, syringoma, photosensitivity, urticaria, hives, warts, wrinkles, callus, acne, athlete’s foot,seborrheic dermatitis, chickenpox, eczema, psoriasis, erythematic, skin aging, contact dermatitis, etc.
Acknowledgements
Authors are grateful to Dabur Research Foundation, Trivedi Global, Inc., Trivedi Science, and Trivedi Master Wellness for their support throughout the work.
References
- 1. Tabata, N., O’Goshi, K., Zhen, Y.X., et al. Biophysical assessment of persistent effects of moisturizers after their daily applications: Evaluation of corneotherapy. (2000) Dermatology 200(4): 308-313.
- 2. Lübbe, J. Evidence-based corneotherapy. (2000) Dermatology 200(4): 285-289.
- 3. Varani, J., Warner, R.L., Gharaee-Kermani, M., et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. (2000) J Invest Dermatol 114(3): 480-486.
- 4. Kockaert, M., Neumann, M. Systemic and topical drugs for aging skin. (2003) J Drugs Dermatol 2(4): 435-441.
Pubmed| Crossref| Others
- 5. Margelin, D., Medaisko, C., Lombard, D., et al. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. (1996) J Invest Dermatol 106(3): 505-509.
- 6. Baumann, L. Skin ageing and its treatment. (2007) J Pathol 211(2): 241-251.
- 7. Schwartz, J.R., Marsh, R.G., Draelos, Z.D. Zinc and skin health: Overview of physiology and pharmacology. (2005) Dermatol Surg 31(7): 837-847.
- 8. Park, K. Role of micronutrients in skin health and function. (2015) Biomol Ther (Seoul) 23(3): 207-217.
- 9. McDaniel, S., Goldman, G.D. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. (2002) Arch Dermatol 138(12): 1593-1596.
- 10. Kivirikko, K.I., Myllyla, R. Post-translational processing of procollagens. (1985) Ann NY Acad Sci 460: 187-201.
- 11. Traikovich, S.S. Use of topical ascorbic acid and its effects on photodamaged skin topography. (1999) Arch Otolaryngol Head Neck Surg 125(10): 1091-1098.
- 12. Barnes, P.M., Powell-Griner, E., McFann, K. et al. Complementary and alternative medicine use among adults: United States, 2002. (2004) Adv Data 343: 1-19.
- 13. Movaffaghi, Z., Farsi, M. Biofield therapies: Biophysical basis and biological regulations. (2009) Complement Ther Clin Pract 15(1): 35-37.
- 14. Yount, G., Patil, S., Dave, U., et al. Evaluation of biofield treatment dose and distance in a model of cancer cell death. (2013) J Altern Complement Med 19(2): 124-127.
- 15. Garland, S.N., Valentine, D., Desai, K., et al. Complementary and alternative medicine use and benefit finding among cancer patients. (2013) J Altern Complement Med 19(11): 876-881.
- 16. Meagher, E.M., Trivedi, M.K., Branton, A., at al. An in vitro study of biofield energy healing based herbomineral formulation for skin protection. (2017) American Journal of Laboratory Medicine 2(2): 13-23.
- 17. Dodon, J., Trivedi, M.K., Branton, A., et al. The study of biofield energy treatment based herbomineral formulation in skin health and function. (2017) American Journal of BioScience. 5(3): 42-53.
- 18. Kinney, J.P., Trivedi, M.K., Branton, A., et al. Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. (2017) American J Life Sci 5(2): 65-74.
- 19. Singh, J., Trivedi, M.K., Branton, A., et al. Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. (2017) American J of Pharmacology and Phytotherapy 2(1): 1-10.
- 20. Trivedi, M.K., Tallapragada, R.M. A transcendental to changing metal powder characteristics. (2008) Met Powder Rep 63(9): 22-31.
- 21. Trivedi, M.K., Nayak, G., Patil, S., et al. Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. (2015) Ind Eng Manage 4: 161.
- 22. Dabhade, V.V., Tallapragada, R.R., Trivedi, M.K. Effect of external energy on atomic, crystalline and powder characteristics of antimony and bismuth powders. (2009) Bull Mater Sci 32(5): 471-479.
Pubmed| Crossref| Others
- 23. Sances, F., Flora, E., Patil, S., et al. Impact of biofield treatment on ginseng and organic blueberry yield. (2013) Agrivita J Agric Sci 35(1): 22-29.
- 24. Lenssen, A.W. Biofield and fungicide seed treatment influences on soybean productivity, seed quality and weed community. (2013) Agricultural Journal 83(3): 138-143.
- 25. Trivedi, M.K., Patil, S., Shettigar, H., et al. Antimicrobial sensitivity pattern of Pseudomonas fluorescens after biofield treatment. (2015) J Infect Dis Ther 3: 222.
- 26. Trivedi, M.K., Patil, S., Shettigar, H., et al. Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. (2015) J Microb Biochem Technol 7(4): 202-205.
- 27. Trivedi, M.K., Patil, S., Shettigar, H., An effect of biofield treatment on multidrug-resistant Burkholderia cepacia: A multihost pathogen. (2015) J Trop Dis 3: 167.
- 28. Patil, S.A., Nayak, G.B., Barve, S.S., et al. Impact of biofield treatment on growth and anatomical characteristics of Pogostemon cablin (Benth.). (2012) Biotechnol 11(3): 154-162.
- 29. Nayak, G., Altekar, N. Effect of biofield treatment on plant growth and adaptation. (2015) J Environ Health Sci 1(2): 1-9.
- 30. Nayak, G., Trivedai, M.K., Branton, A. et al. Antiaging Potential of Consciousness Energy Healing-Based Novel Proprietary Formulation. (2018) FDNJ.
- 31. Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (ISO 10993-5:2009), I.S.EN ISO, 10993-5: 2009.
Pubmed| Crossref| Others
- 32. Hahn, M.S., Kobler, J.B., Starcher, B.C., et al. Quantitative and comparative studies of the vocal fold extracellular matrix. I: Elastic fibers and hyaluronic acid. (2006) Ann Otol Rhinol Laryngol 115(2): 156-164.
- 33. Zhang, L., Yoshida, T., Kuroiwa, Y., et al. Stimulation of melanin synthesis of B16-F10 mouse melanoma cells by bufalin. (1992) Life Sci 51(1): 17-24.
- 34. Wen, K.C., Shih, I.C., Hu J.C., et al. Inhibitory effects of Terminalia catappa on UV-B-induced photodamage in fibroblast cell line. (2011) Evid Based Complement Alternat Med.
- 35. Fronza, M., Heinzmann, B., Hamburger, M., et al. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. (2009) J Ethnopharmacol 126(4): 463-467.
- 36. Yadav, K., Singhal, N., Yadav, H., et al. Cell proliferation assays. (2014)
- 37. Albini, A., Adelmann-Grill, B.C. Collagenolytic cleavage products of collagen Type I as chemoattractants for human dermal fibroblasts. (1985) Eur J Cell Biol 36(1): 104-107.
- 38. Jeffrey J Metalloproteinases and tissue turnover. Wounds (1995) 7: 13-22.
Pubmed| Crossref| Others
- 39. Almine, J.F., Wise, S.G., Weiss, A.S. Elastin signaling in wound repair. (2012) Birth Defects Res C Embryo Today 96(3): 248-257.
- 40. Yin, L., Morita, A., Tsuji, T. Skin aging induced by ultraviolet exposure and tobacco smoking: evidence from epidemiological and molecular studies. (2001) Photodermatol Photoimmunol Photomed 17(4): 178-183.
Pubmed| Crossref| Others
- 41. Castelo-Branco, C., Figueras, F., Martínez d.e Osaba, M. J., et al. Facial wrinkling in postmenopausal women. Effects of smoking status and hormone replacement therapy. (1998) Maturitas 29(1): 75-86.
- 42. Youn, C.S., Kwon, O.S., Won, C.H., et al. Effect of pregnancy and menopause on facial wrinkling in women. (2003) Acta Derm Venereol 83(6): 419-424.
- 43. Contet-Audonneau, J.L., Jeanmaire, C., Pauly., G. A histological study of human wrinkle structures: Comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. (1999) Br J Dermatol 140(6): 1038-1047.
- 44. Brincat, M., Moniz, C.J., Studd, J.W., et al. Long-term effects of the menopause and sex hormones on skin thickness. (1985) Br J Obstet Gynaecol 92(3): 256-259.
- 45. Frantz, C., Stewart, K.M., Weaver, V.M. The extracellular matrix at a glance. (2010) J Cell Sci 123(24): 4195-4200.












