Faster Recovery from Isoflurane N2O/O2- Anesthesia Using Quartz Generated, High Frequency Low Energy Sinusoidal Waves - A Randomized Placebo Cross-Over Study in Rats
Enno Freye1*, Joseph, Victor Levy2
Affiliation
- 1Department of Vascular Surgery and Renal Transplantation, Heinrich-Heine University Clinics of Duesseldorf/Germany
- 2Department of Pharmacology & Physiology, University of the Pacific Dental School Webster Street, San Francisco, California/USA
Corresponding Author
Enno Freye, MD, PhD Färbistr.3, 7270 Davos-Platz, Switzerland, E-mail: enno.freye@uni-duesseldorf.de
Citation
Freye, E., et al. Faster Recovery from Isoflurane N2O/O2-anesthesia using Quartz generated, High-Frequency Low Energy Sinusoidal Waves - A Randomized Placebo Cross-Over Study in Rats. (2016) J Anesth Surg 3(1): 85-89.
Copy rights
©2016 Freye, E. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Isoflurane-anesthesia; Electroencephalogram; Sinusoidal electromagnetic waves; Recovery rate from anesthesia.
Abstract
The present study was undertaken to prove if the presently dominant quantitative “structure-activity relationship” theory of molecular signaling by means of chemical/physical binding, should rather be replaced by information processing using electromagnetic wave transmission.
Rats after a steady-state isoflurane N2O/O2-anesthesia were exposed to electromagnetic waves and the speed of recovery measured at regular intervals determining nociception, balancing on a rotary rod, and vigilance (EEG). Compared to recovery rate without exposure to electromagnetic waves the same animals demonstrated no difference in the return of nociception, a significant (p < 0.05) faster ability for balance on a rotary rod and a highly significant (p < 0.005) faster return in higher cortical centers and vigilance.
These preliminary data underline the connotation, that recovery from anesthesia can be hastened by means of sinusoidal electromagnetic waves possible via activation of mitochondrial ATP turnover.
Introduction
Rapid reversal of the depressive effects of anesthesia and the recovery of reflex control after ambulatory surgery is of paramount importance for the patient in order to regain consciousness and reflex activity, to ambulate and shorten the period in the PACU (post anesthesia care unit). When using volatile anesthetics, recovery from anesthesia is accomplished by means of a decline in the concentration equilibrium within the central nervous system, which is dependent from a decline of concentration within the vascular compartment and corresponds with concentration within the alveoli. While an increase in the respiratory rate may to a certain degree results in a faster decline in the concentration of the anesthetic in different compartments, this is limited by the blood/gas equilibration ratio of the anesthetic. And although the introduction of short acting volatile agents of the newer generation such as desflurane and sevoflurane has markedly shortened recovery time[1,2], there is, however, still a demand to shorten this period. Especially, by regaining reflex control, patients are able to ambulate earlier after anesthesia, there is a lesser incidence in morbidity and side-effects, especially PONV and respiratory depression[3].
Since previous in-vitro data have demonstrated that cells emit special infrared spectra[4], it was tested if normalization of neuronal cell function, after being exposed to a volatile anesthetic, can be hastened by applying electromagnetic waves. Such assumption was derived from data of other researchers, who conclusively demonstrated an activation of specific cell functions by corresponding low frequency (> 20 kHz) electromagnetic waves[5,6]. We therefore set out to test this hypothesis in the whole animal comparing recovery rates with a placebo-controlled group. By exposing rats to quartz-generated pulsed high frequency low energy sinusoidal waves after the volatile anesthetic isoflurane, it was tried to reverse the cortical functional deficits while at the same time evaluating changes in electroencephalographic (EEG) activity.
Materials and Methods
Following approval by the local Committee for Animal Research of the county of Northrhein-Westfalia, 15 male wistar rats were incorporated in the experimental design. With a mean weight of 317 g (± 20 SD), and a previous free access to water and pellet feeding ad lib, they were retained from food the night before the experiment. On the next morning they were placed into a plexiglas chamber which was attached to the hose of an open Q-circle anesthesia machine (Sulla 800V®, Dräger company Lübeck/Germany) and exposed to a step-wise increase in concentrations of the volatile anesthetic isoflurane in a nitrous oxide/oxygen mixture (3:1) until they lost consciousness and did not respond to clamping of the base of the tail with a hemostat (MAC 95). The anesthetic was delivered via a vaporizer (Isoflurane Dräger Vapor, model 19.3 from Dräger company Lübeck/Germany) and after having attained a steady-state anesthetic/analgesic concentration, anesthesia was maintained for 30 minutes.
For evaluation of anesthetic depth, two Ag/AgCl electrodes were pasted to the scalp and the derived electroencephalographic signals fed into a portable computerized EEG machine (Lifescan®, Diatek company, San Diego, USA) which by means of Fast Fourier Transformation (FFT) computed the spectral edge frequency (SEF 95)over a time epoch of 60 sec, comprising 95% of power of all electroencephalographic waves within the typical EEG spectra alpha, beta, theta and delta.
For measurement of respiratory rate the inspiratory port of the anesthesia Q-circle was hooked up to a Datex Capnomac® endexspiratory CO2-measurement system (Datex company, Helsinki, Finland), which determined endexspiratory isoflurane concentration while integrating the breaths/min. By thus it was possible to determine respiratory cycles and the isoflurane concentrations necessary to induce analgesia in the animals and the concentration of isoflurane at the end of anesthesia after which the animals were exposed to room air.
Immediately following anesthesia animals were placed between two coils, which were hooked up to the control unit of the Coufal electromagnetic device (Coufal Energy Production Inc, Wolfhalden/Switzerland), which was set to continuously deliver quartz generated high frequency(> 6 MHz), amplitude-modulated low energy (> 1 μWatt/cm²) sinusoidal waves. In order to determine the state of recovery and the level of cortical function depression, animals after anesthesia underwent different functional tests every 15 s and the corresponding reaction time recorded evaluating different level of anesthetic action:
The time span of reaction to clamping the base of the tail with a hemostat (= recovery of reflex arch of sensory mediation within the spinal cord).
The time span being able to balance on a rotating rod (= recovery of muscular spinal-related writhing reflex).
The time span starting to explore the environment by sniffing and walking (= thalamo-cortical reactivation and an increased state of vigilance).
Following a period of at least two weeks, they same animals were exposed to the same protocol this time, however, without exposure to quartz generated pulsed high frequency, low energy sinusoidal waves while determining the time span for recovery of different levels of higher cortical functions.
In addition, the ATP turnover was also measured in rat granulocytes before and after after sinusoidal waves in both groups in order to determine if ATP synthesis could play role in the effect of any kind of change in recovery rate (Biovis Laboratory for Diagnostics, Limburg/Germany).
Statistical analysis
For the detection of statistical significance between the two groups regarding difference in reaction times and assuming approximate Gaussian distribution, the two-tailed paired t-test was used.
A value of p < 0.05 was considered statistically significant.
Results
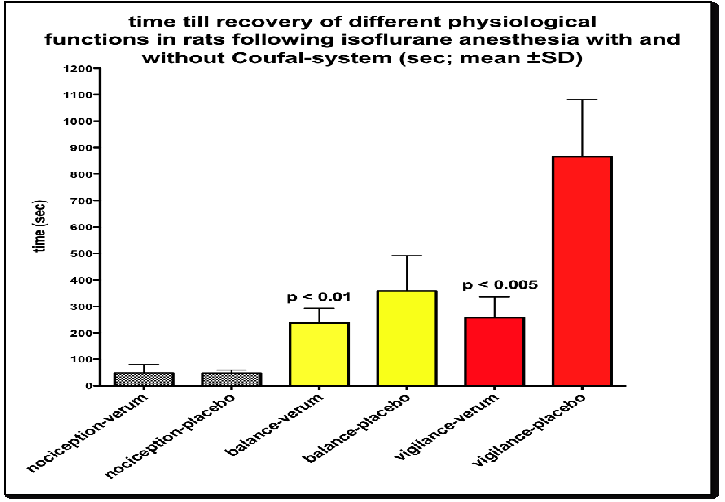
Mean induction of anesthesia to achieve total blockade of nociceptive reflex was 516.5 s (± 78 SD) in the verum and 480 s (± 114 SD) in the placebo group. Although the time span for reaching steady state anesthesia was faster in the placebo group, this was statistically not significant (Table 1). The concentration of the anesthetic, necessary to generate analgesia in both groups, i.e. unresponsiveness to clamping the base of the tail with a hemostat, endexspiratory concentration of isoflurane was 1.48 vol% (± 0.04 SD) in the verum and 1.54 vol% (± 0.06 SD) in the placebo group (Table 1; ns).
Table 1: Cortical activity as reflected in spectral edge frequency, respiratory rate, time for induction of anesthesia and the necessary isoflurane concentration in N2O/O2 (3:1) to induce antinociception in both groups of animals, with and without use of electromagnetic waves (mean ± SD) SEF= spectral edge frequency of the EEG.
| Parameters | Control-group | Verum-group |
|---|---|---|
| Induction time till unresponsiveness (s) | 490.0 ± 114 | 516.5 ± 78 |
| respiratory rate at end of anesthesia (cycles/min) | 75 | 69 |
| EEG spectral edge frequency at steady state anesthesia (Hz) | 13 | 13.5 |
| EEG spectral edge at point of recovery of higher cortical functions (Hz) | 18 | 21* |
| endexspir. isoflurane concent. (vol%) with unresponsiveness to stimulus | 1.54 ± 0.06 | 1.48 ± 0.04 |
| Recovery of nociceptive reflex (s) | 48.5 ± 9.5 | 49.0 ± 20 |
| Recovery of gross muscular strength & balance (s) | 358 ± 100 | 238 ± 25* |
| recovery of grooming, sniffing & exploration of environment (s) | 866 ± 300 | 258 ± 45** |
Mean ± SD; two tailored paired t-test *p < 0.01; **p < 0.005
Table 2:
| Basal ATP-turnover rate within granulocytes | ATP recovery rateafter isoflurane without sinusoidal waves | ATP recovery rate after isoflurane with sinusoidal waves |
| 390 ± 50 pmol/106 cells | 110 ± 20** pmol/106 cells | 550 ± 60|| pmol/106 cells |
Mean ± SEM; ANOVA. Significance compared to basal ATP turnover rate ** p < 0.001. Significance following administration with and without sinusoidal waves ||p < 0.001
Following a maintenance period of anesthesia for 30 min, EEG spectral edge frequency (SEF) was 14.8 Hz in the verum and 14.5 Hz in the placebo group at the end of anesthesia (ns). In addition to a similar cortical depression as being visualized in the computerized EEG, respiratory rate in both groups was statistically not different at the end of the anesthesia period (69 cycles/min in the verum versus 75 cycles/min in the placebo group; Table 1).
With a mean isoflurane concentration of 1.48 vol% and 1.45 vol% (in N2O/O2) respectively and after 30 min of steady state anesthesia, mean time for the recovery of the nociceptive reflex was 49 s (± 20 SD) in the verum and 48.5 s (± 7.5 SD) in the placebo group (ns). These data indicate, that in spite administration of electromagnetic waves, return of nociception was similar in both groups.
In contrast, mean time to regain ability for balance on a rotatory rod was faster in the verum compared to the placebo group (238 s versus 358 s; Figure. 1). Such faster return of the animal’s ability for regaining balance on a rotating rod was significant (p < 0.05) favoring the use of the specific electromagnetic waves for reversal of depression. But more so, recovery rate between both groups was more pronounced and shortened in regard to the return of vigilance. This was demonstrated by a faster return of spontaneous activity to explore the environment. By using the electromagnetic waves, meantime span till recovery of such higher cortical function (grooming, sniffing, exploration of environment) was 258 s in the verum and 866 s in the placebo group. These data demonstrate a highly significant difference (p < 0.001, Figure. 1) and a 3-fold faster return of higher cortical functions, as being visualized in the SEF of the EEG with values of 19 Hz in the control and 21 Hz in the verum group. In addition to behavioral and EEG-data, ATP concentrations, as being measured in rat granulocytes, demonstrated a significant (p > 0.001) higher turnover in only those rats having been exposed to sinusoidal waves (Table 2).
Figure 1: Difference in recovery times (mean ± SD in s) following exposure to isoflurane-N2O/O2 for 30 min, with and without the use of amplitude-modulated waves. No difference in regard to recovery of nociception, while improvement in balance and vigilance is significant between the two groups.
Discussion
High-frequency electromagnetic field waves presently are being discussed repetitively to induce effects on biological systems. Field waves, similar to those used by communication telephones, have been demonstrated to induce firing rate of neurons of the CNS by 3-5fold[7]. In addition, cognitive-protective and cognitive enhancing effects, both in normal and transgenic mice destined to develop Alzheimer’s-like cognitive impairment, was demonstrated by others when using high-frequency electromagnetic field (EMF) waves[8]. In regard to such results, it is not too surprising that the presently applied sinusoidal, amplitude- modulated electromagnetic waves were able to reverse some the depressive effects of an isoflurane/N2O anesthetic mixture on cortical functions. However, in contrast to the protective and cognitive enhancing effect of electromagnetic waves in transgenic mice, the presently used waves do not contain rectangular, pulsed components such as being used in a mobile phone. They are of sinusoidal character and have aquartz-generated ultrahigh frequency of 7.14 megahertz, comprising amplitude-modulated low energy of harmonic nature. Such waves enclose the energy spectrum of a healthy organism, which therefore are able to induce resonance in an organism. And since initiation of resonance is able to induce recovery of a depressed neuronal network, such mechanism of action seems to be a necessary part to increase the recovery process from anesthesia. Thus, only when resonance is evident, neuronal cells have the ability to regain regular, unsuppressed function.
In practice, reversal of volatile anesthesia is achieved by means of shutting off the vaporizer, which is followed by a drop of the inspiratory concentration of the anesthetic and a decline of the concentration gradient within the CNS followed by gradual recovery of higher cortical functions. Our data demonstrate, that such recovery can be accelerated simply by the application of sinusoidal amplitude-modulated waves and without the use of an intravenous agent. As to the mode of action of such increase of firing rate of cortical cells as demonstrated in the EEG, an increase of spin and frequency of atoms and electrons within the mitochondria of cells, as previously demonstrated by others[9], is a likely explanation. Such an explanation is underlined by others who had demonstrated in isoflurane-anesthetized mice and by using a similar evaluation scale as in our experiment, that the anesthetic effect was primarily induced via blockade of complex I within the mitochondria, the most relevant site of synthesis of ATP maintaining sufficient cellular function[10]. Thus, externally applied waves, aside from a possible increased rate in ATP-synthesis, augment the electronic spin and oscillation within atoms of lipid membranes resulting in a faster return of cellular function. Such proposed mechanism of action is underlined by unpublished results using the same systems in human volunteers. There, continuous EEG monitoring was able to demonstrate conclusively, that in comparison to the dominant alpha activity within the 7-13 Hz band during the awake and relaxed state, externally applied sinusoidal waves induce a shift of the dominant EEG power towards the beta band (13-30 Hz) activity, an effect, which is characterized by a desynchronization of EEG waves and a higher frequency rate. Since such shift corresponds with a higher state of vigilance[11,12], it also goes in hand with a focused response to environmental stimuli, i.e., an increase in wakefulness. Such assumption is corroborated by the present EEG power spectra, which had been derived from the cortex in our animal study. From a spectral edge frequency with a mean value of 14 Hz at the end of anesthesia, frequency increased to a mean of 19 Hz. This higher frequency corresponded with an increase in wakefulness and the resumption of the aptitude to explore a new environment[13], reflecting an increase in vigilance, which very much can be explained by the more pronounced ATP turnover as demonstrated in granulocytes of the same animals (Table 2).
Therefore, the commonly accepted mode of action of volatile anesthetics by means of an unspecific loss in membrane potentials within lipid membranes, followed by a deficit in activity with a ensuing loss of consciousness and nociception[14], has to be extended. The most likely explanation is the activation of ATP-synthesis within the mitochondria of neuronal cells by means of sinusoidal waves, resulting in a faster return of normal activity and a faster return of wakefulness. This assumption is further underlined by results from cell-culture studies where general anesthetics significantly inhibited mitochondrial ATP-synthesis[15,16], but also by our own data in rat granulocytes which demonstrates a significant higher turnover in ATP synthesis following application of sinusoidal waves.
Although recovery of a very basic reflex behavior, the response to a nociceptive stimulus, did not demonstrate any significant difference in animals with and without exposure to sinusoidal waves, higher cortical functions such as balancing on a rotary rod and vigilance demonstrated a faster recovery process. Especially, the recovery to a point of exploring the environment is only possible by means of a recuperation of higher cortical functions from anesthesia. Since such faster recovery was only seen in animals being exposed to sinusoidal waves, this also was reflected by a shift of EEG powers spectra from 14 to 21 Hz at the moment of spontaneous movement. In this regard the electromagnetic waves may act as a booster of CNS activity once they get into resonance resulting in a faster recovery after a volatile anesthetic, an important prerequisite as already pointed out by others[17].
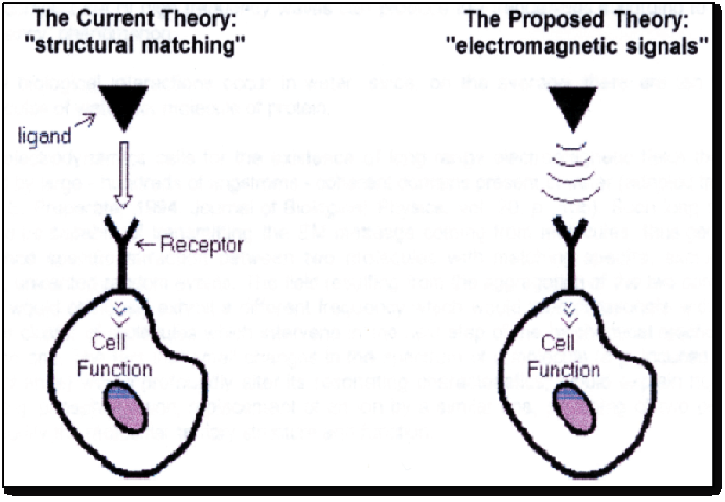
From the underlying data, a new hypothesis is put forward that anesthetics may act on neuronal cells, where receptors sites act like small antennas (Figure. 2) at which the electrical sinusoidal waves induce a quantum mechanic and life-inducing turnstile rotation of molecules as demonstrated by other researchers[18]. Such action in return induces a transmembrane phosphorylation of ADP to ATP within the mitochondria resulting in a consequential increase of the necessary fuel to run neuronal cells. While contemporary pharmacology of anesthetics teaches that activity between neuronal cells is regulated via receptor binding and transmitter flow, such conventional way of thinking nowadays is being questioned. This is because microwaves, acoustic waves as well as recently discovered scalar waves regulate morphogenesis, function of cells, hormonal release, cellular mitosis, as well as nerve fiber growth[19-21]. In addition, electromagnetic waves have also been shown to increase the rate of phosphorylation within the cell[22], underlining the cause for the increase in neuronal activity and the faster recovery of vigilance in the present study. Presently, it is still unclear if the applied pulsed sinusoidal waves induce a state of resonance in neuronal cells, which coincides with a faster recovery of higher cortical functions. However, the hypothesis that electromagnetic frequencies are able to transmit information at a much higher speed than any biochemical signal may it be a hormone or a transmitter[23] is being increasingly accepted by researchers.
Figure 2: The proposed mechanisms of action of electromagnetic waves on cellular function.
Therefore, the presently dominant quantitative “structure-activity relationship” theory of molecular signaling, which claims that two structurally matching molecular species exchange specific information by chemical/physical binding, also referred to as the key/lock interaction model, is being questioned repetitively. This is because such specific molecular interactions occur only after random collisions between a molecule and a “receptor”, involving electrostatic, short-range forces, which takes too much time in order to result in an immediate reaction. Such a paradox is still unexplained by those researchers adhering to the widespread theory of information processing. The shortcomings of this approach are illustrated by the widely recognized failure of “drug design” to produce an expected stereochemical match with the target “receptor”. Also, in this context it is worth mentioning that the words “molecular signal” are routinely used by biologists, yet they do not give a precise physical definition when it comes to the mediation of an effect.
In conclusion, the above data underline the need for a change in the way of thinking how agents in general and volatile anesthetics in particular act on neuronal activity. And although extensive research is still necessary to completely understand, how sinusoidal waves interact with biological membranes, the above study clearly demonstrates that such waves may be of clinical benefit.
Acknowledgment
The researches gratefully acknowledge the skilled technical support of Miss A. Schrey, veterinary assistance. In addition, we thank HP Coufal of Coufal Energy Production Inc. in Wolfhalden/Switzerland for supply of the electromagnetic device. The research was conducted in the Central Animal Laboratory Unit of the University of Dusseldorf and was not founded by any sponsor.
†In memory of Prof. Joseph Victor Levy, former head of the Dept. of Pharmacology/Physiology at the University of the Pacific/San Francisco, a dear friend, mentor and colleague who had passed away in 2009.
References
- 1. Ekman, A., Brudin, L., Sandin, R. A comparison of bispectral index and rapidly extracted auditory evoked potentials index responses to noxious stimulation during sevoflurane anesthesia. (2004) Anesth Analg 99(4): 1141-1146.
- 2. Freye, E., Bruckner, J., Latasch, L. No difference in electroencephalographic power spectra or sensory-evoked potentials in patients anaesthetized with desflurane or sevoflurane. (2004) Eur J Anaesthesiol 21(5): 373-378.
- 3. Sneyed, J.R., Carr, A., Byrom, W.D., et al. A meta-analysis of nausea and vomiting following maintenance of anaesthesia with propofol or inhational agents. (1998) Eur J Anaesthesiol 15(4): 433-445.
- 4. Mourant, J.R., Gibson, R.R., Johnson, T.M., et al. Methods for measuring the infrared spectra of biological cells. (2003) Phys Med Biol 48(2): 243-257.
- 5. Davenas, E., Beauvais, F., Amara, J., et al. Human basophil degranulation triggred by very dilute antiserum against IgE. (1988) Nature 333(6176): 816-818.
- 6. Benveniste, J., Aissa, J., Guillonnet, D. A simple and fast method for in vivo demonstration of electromagnetic molecular signaling via high dilution or computer recording . (1999) FASEB J 13(4): A163.
- 7. Beason, R.C., Semm, P. Responses of neurons to an amplitude modulated microwave stimulus. (2002) Neurosci Lett 333(3): 175-178.
- 8. Arendasha, G.W., Sanchez-Ramos, J., Mori, T., et al. Electromagnetic Field Treatment Protects Against and Reverses Cognitive Impairment in Alzheimer’s Disease Mice. (2010) J Alzheimer Dis 19(1): 191-210.
- 9. Huping, H., Maoxin, W. Spin as primordial self-referential process driving quantum mechanics, spacetime dynamics and consciousness. (2004) Neuro Quantology 2(1): 41-49.
- 10. La Monaca, E., Fodale, V. Effects of Anesthetics on Mitochondrial Signaling and Function. (2012) Curr Drug Saf 7(2): 126-139.
- 11. Freye, E. Cerebral monitoring in the operating room and the intensive care unit - an introductory for the clinician and a guide for the novice wanting to open a window to the brain. Part II: Sensory-evoked potentials (SSEP, AEP, VEP). (2005) J Clin Monit Comput 19(1-2): 77-168.
- 12. Freye, E., Levy, J.V. The effects of tramadol on pain relief, fast EEG-power spectrum and cognitive function in elderly patients with chronic osteoarthritis (OA). (2006) Acute Pain 8(2): 55-61.
- 13. Dijk, D.J., Daan, S. Sleep EEG spectral analysis in a diurnal rodent: Eutamius sibiricus. (1989) J Comp Physiol A 165(2): 205-215.
- 14. Marder, E., Abbott, L.F., Turrigiano, G.G., et al. Memory from the dynamics of intrinsic membrane currents. (1996) Proc Natl Acad Sci U S A 93(24): 13481-13486.
- 15. Kohro, S., Hogan, Q.H., Nakae, Y., et al. Anesthetic effects on mitochondrial ATP-sensitive K channel. (2001) Anesthesiology 95(6): 1435-14340.
- 16. Zaugg, M., Pasch, T., Garcia, C., et al. Differential effects of anesthetics on mitochondrial K(ATP) channel activity and cardiomyocyte protection. (2002) Anesthesiology 97(1): 15-23.
- 17. Helmuth, L. Neuroscience. Boosting brain activity from the outside in. (2001) Science 292(5520): 1284-1286.
- 18. Pophristic, V., Goodmann, L. Hyperconjugation not steric repulsion leads to the staggred structure of ethane. (2001) Nature 411: 565-568.
- 19. Liboff, A.R. Toward an electromagnetic paradigm for biology and medicine. (2004) J Altern Complement Med 10(1): 41-47.
- 20. Goodmann, R., Blank, M. Insights into electromagnetic interaction mechanisms. (2002) J Cell Physiol 192(1): 16-22.
- 21. Sivitz, I. Cells proliferat in magnetic fields. (2000) Science News 158(13): 195.
- 22. Jin, M., Blank, M., Goodman, R. ERK1/2 phophorylation induced by electromagnetic fields, diminishes during neoplastic transformation. (2000) J Cell Biochem 78(3): 371-379.
- 23. McClare, C.W. Resonance in bioenergetics. (1974) Ann NY Acad Sci 227: 74-97.