Fasting C-Peptide Index May be the Strongest Predictor for Detecting Effectiveness of Glycemic Control by GLP-1 Analogue, Liraglutide Therapy for Japanese Type 2 Diabetic Patients
Yoko Matsuzawa, Takumi Kitamoto and Tetsuo Nishikawa*
Affiliation
Endocrinology and Diabetes Center, Yokohama Rosai Hospital, Yokohama, Japan
Corresponding Author
Tetsuo Nishikawa, Endocrinology and Diabetes Center, Yokohama Rosai Hospital, 3211 Kozukue-cho, Kohoku-ku, Yokohama City, Kanagawa 222-0036, Japan. Tel: +81-45-474-8111; Fax: +81-45-474-8323; E-mail: tetsuon@yokohamah.rofuku.go.jp
Citation
Tetsuo, N., et al. Fasting C-Peptide Index May be the Strongest Predictor for Detecting Effectiveness of Glycemic Control by GLP-1 Analogue, Liraglutide Therapy for Japanese Type 2 Diabetic Patients. (2014) J Diabetes Obes 1(1): 27-30.
Copy rights
© 2014 Tetsuo N. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Glucagon-like peptide-1 receptor agonist; Type 2 diabetes; C peptide, Eating behavior
Abstract
Liraglutide (Victoza, Novo Nordisk Pharma Ltd.), a GLP-1 analogue preparation for diabetes mellitus has been reported to potently reduce the blood sugar level and decrease the body weight when injected once a day. As the risk of hypoglycemia is low, it has been used as an epoch-making drug in a rapidly increasing number of patients. In this study, we investigated the effects of liraglutide subcutaneously injected once a day in 48 patients with type 2 diabetes who had been treated at the outpatient clinic of the Diabetes Center in Yokohama Rosai hospital without admission. After 3 - month administration, the mean HbA1c value was significantly decreased (from 8.61 to 8.04%), and the mean body weight was also significantly reduced (from 71.0 to 69.2 kg). However, there were marked individual differences in the effects. We compared background factors between groups with and without improvement. The results showed that liraglutide was particularly effective in patients with a short duration of diabetes in whom endogenous insulin secretion, which was evaluated by examining fasting C-peptide with fasting sugar level, was well maintained. Furthermore, we examined the characteristics of eating behavior before and after administration using a questionnaire. In patients with a significant decrease in the blood sugar level, there was a marked improvement in the eating behavior pattern. Therefore, not only a decrease in the blood sugar level related to improved ability of endogenous insulin secretion but also the improvement of a patient\'s eating behavior was closely associated with a decrease in the blood sugar level after liraglutide administration.
Introduction
It is well known that the glucagon-like peptide-1 receptor agonists, exenatide, liraglutide and lixisenatide, can improve glycemic control without inducing hypoglycemia in type 2 diabetes. Liraglutide (Victoza, Novo Nordisk Parma Ltd.), a GLP-1 analogue preparation for diabetes mellitus, which became commercially available in Japan in 2010, has been reported to potently reduce the blood sugar level and decrease the body weight when injected once a day. As the risk of hypoglycemia is low, it has been used as an epoch-making drug in a rapidly increasing number of patients[1]. It may also reduce body weight[2]. However, these effects have not been sufficiently verified in clinical practice, and the influence of this drug on eating behavior also remains to be clarified. In this study, we compared type 2 diabetics eating behavior before and after the administration of this drug to obtain findings regarding its appropriate and effective use.
Subjects and Methods
We selected 48 patients who had been suffered from type 2 diabetes for more than 10 years, receiving a long time (more than 1 year) complex insulin therapy or several oral anti-diabetic agents, including glimepiride and metformin, and they were poorly controlled with high HbA1C (>8.0%) for at least past 6 months in the outpatient clinic of the Diabetes Center in our hospital. Liraglutide, a GLP-1 analogue preparation, which was used by replacing insulin or simultaneously adding to the same treatment as previously administrating oral hypoglycemic agents, was subcutaneously injected once a day (0.9mg/day) in those patients at the outpatient clinic. The following parameters were compared before and after treatment:
1) We did assessment of the blood sugar control by examining fasting blood sugar level with fasting C-peptide and HbA1c. Fasting C - peptide index was calculated as the ratio of fasting C - peptide (ng/ml) to fasting blood sugar level (mg/dl) (X100).
2) We performed survey regarding eating behavior by using "Questionnaire Regarding Eating Behavior" from the Japan Society for the Study of Obesity questionnaire[3,4]. In a questionnaire consisting of 55 items, answers must be given in 4 grades to questions on "Recognition for weight and constitution", "External eating behavior", "Emotional eating behavior", "Sense of hunger", "Eating style", "Food preference", and "Regularity of eating".
The present study was approved by the research ethics committee of Yokohama Rosai Hospital, and all of the subjects understood the objectives of the study and gave their written consent.
Statistical analysis
Data are presented as the mean ± SD. Comparisons were performed using the paired t-test. We considered P values of ≤ 0.05 to be significant. Multivariate forward stepwise linear regression analysis was performed as shown in Table 3. Statistical analyses were performed using Excel 2010 statistical software package (version 1.10. Tokyo, Japan).
Table 1: Clinical characteristics of subjects
| Total (n = 48) | (n = 25) | female (n = 23) | p value | |
|---|---|---|---|---|
| age(years old) | 54.8± 13.1 | 52.6 ± 14.3 | 57.2 ± 11.4 | 0.234 |
| duration of diabetes (years) | 12.0 ± 6.8 | 12.3 ± 6.5 | 11.7 ± 7.2 | 0.802 |
| body mass index (Kg/m2 ) | 27.5 ± 4.4 | 26.4 ± 4.0 | 28.7 ± 4.5 | 0.080 |
| HbA1c before Starting liraglutide(%) | 8.6 ± 1.3 | 8.5 ± 1.3 | 8.7 ± 1.3 | 0.542 |
| frequency of insulin usage before starting liraglutide (%) | 77.1 | 76.0 | 78.3 | 0.852 |
| total amount of insulin dosage(unit/day) | 20.3 ± 11.3 | 21.1 ± 12.0 | 20.3 ± 11.8 | 0.870 |
Results
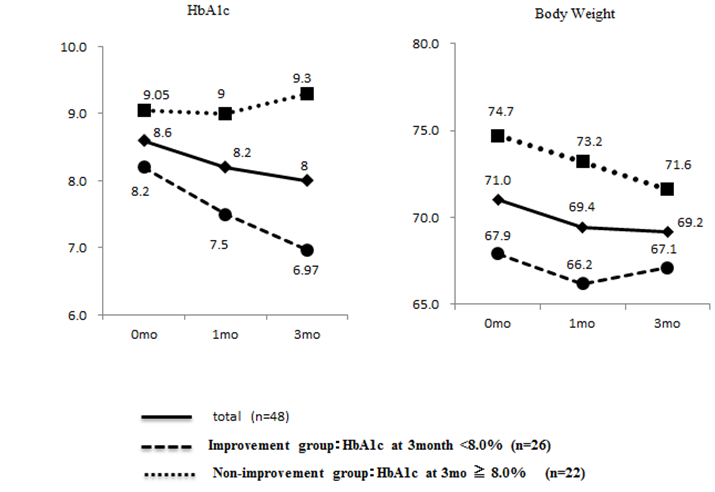
The background of the subjects is shown in Table 1. The mean age was 54.8 ± 13.1 years (mean ± SD). The mean duration of disease was 12.0 ± 6.8 years. The mean HbA1c value was 8.6 ± 1.3%. The mean body mass index (BMI) was 27.5 ± 4.4, suggesting slight obesity. In 77.1% of the subjects, insulin had been used. There were no gender differences in these background factors. The mean HbA1c value significantly decreased (from 8.61 to 8.04%) during a 3 - month period after the start of liraglutide administration, and the mean body weight also significantly reduced (from 71.0 to 69.2 kg) (Figure 1).
Figure1: Changes of HbA1c and Body Weight Changes of HbA1c (%) and body weight (Kg) before and 1or 3 months after starting liraglutide in type 2 diabetes, as described in detail in the text.
However, there were marked individual differences in the patient's responses. Patients with an HbA1c value of 7.9% or lower 3 months after the start of liraglutide administration in whom an improvement was achieved in comparison with the pretreatment value were assigned to an improvement group (n = 26), and those who did not meet these conditions were assigned to a non-improvement group (n = 22). We analyzed the characteristics of the two groups. Moreover, we followed them up for 6 and 12 months after injecting liraglutide. We could treat 39 and 25 subjects among 48 cases, to whom liraglutide started to use for the present study, for 6 and 12 months, respectively. The cases of the improvement group at 3 - month treatment with liraglutide still showed well-controlled HbA1C (26 cases of the improvement group; 7.6 ± 1.2% vs. 13 cases of the non - improvement group; 9.0 ± 0.9, p = 0.002) at 6 - month treatment and (21 cases of the improvement group; 7.3 ± 1.0% vs. 4 cases of the non-improvement group; 9.2 ± 1.8, p = 0.008) 12 - month treatment, respectively. The main reasons why liraglutide - treatment discontinued in the drop - out cases were the adverse effect of gastrointestinal symptoms and no significant effect on reducing HbA1C in the non-improvement group.
Table 2: Comparison of clinical profies of subjects with or without improvement in glycemic control after treatment with liraglutide
| Total (n = 48) | improvement group (n = 26) | non-improvement group(n = 22) | p value | |
|---|---|---|---|---|
| age (years old) | 54.8 ± 13.1 | 54.4 ± 12.7 | 55.2 ± 13.8 | 0.825 |
| duration of diabetes (years) | 12.0 ± 6.8 | 10.2 ± 5.8 | 14.2 ± 7.3 | 0.038 |
| BMI before starting liraglutide | 27.5 ± 4.4 | 26.2 ± 4.2 | 29.0 ± 4.1 | 0.025 |
| HbA1c before starting liraglutide(%) | 8.6 ± 1.3 | 8.2 ± 1.0 | 9.1± 1.5 | 0.025 |
| CPR index before starting liraglutide | 1.35 ± 0.82 | 1.67 ± 0.74 | 1.07± 0.81 | 0.023 |
| CPR index after treatment | 2.33 ± 1.96 | 3.30 ± 2.34 | 1.41 ± 0.81 | 0.002 |
| total amount of insulin dosage (unit/day) | 20.3 ± 11.3 | 14.5 ± 8.1 | 27.2 ± 10.7 | 0.000 |
The clinical background of the improvement and non-improvement groups is presented in (Table 2). In the improvement group, the duration of disease was shorter than in the non-improvement group, and the BMI was lower. The HbA1c value before administration was lower. The CPR index before and after administration was higher, and the total dose of insulin in insulin-treated patients was lower. On double regression analysis, the "dose of insulin" and "CPR index before administration" was selected as significant variables (Table 3), while HbA1C levels before starting liraglutide could not directly affect the effectiveness of liraglutide.
Table 3: Multiple regression analgsis of various parameters inflencing HbA1c values after treatment with liraglutide (multiple correlation coeffiient; R = 0.751) CPR index; fasting c-peptide index
| Partial regression coeffiient | Standard error | Standardised partial regression coeffiient | Standardised partial regression coeffiient | |
|---|---|---|---|---|
| total amount of insulin dosage | 0.0630 | 0.0181 | 0.4943 | 0.0019** |
| CPR index before starting liraglutide | - 64.9122 | 24.6570 | - 0.3969 | 0.0146* |
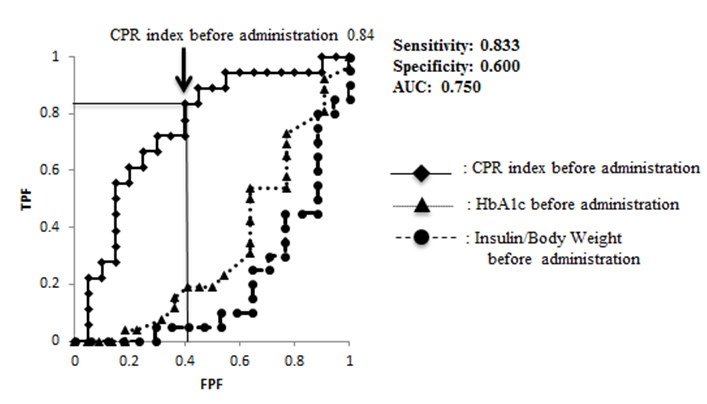
ROC analysis was performed, regarding an HbA1c value of 7.9% or lower achieved after administration as a reference. The sensitivity and specificity of the CPR index before administration were 0.833 and 0.600, respectively, being the highest (Figure 2).
Figure 2: ROC analysis about the achievement of HbA1c < 8.0% ROC analysis was performed, regarding an HbA1c value of 7.9% or lower achieved after administration as a reference.
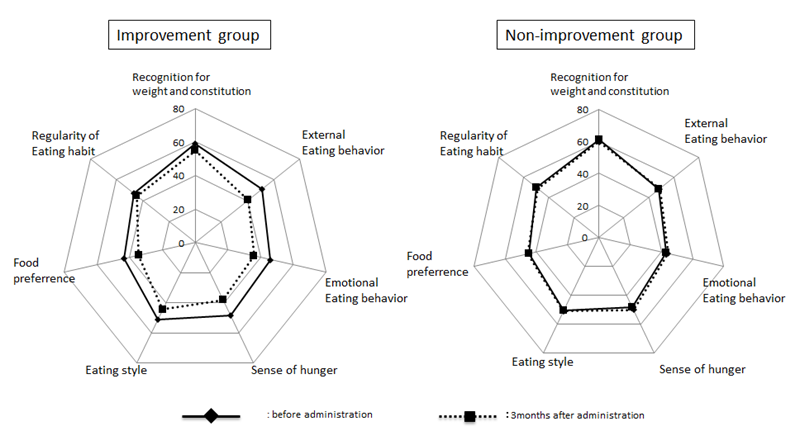
In this study, we conducted a questionnaire survey regarding eating behavior before and after liraglutide administration, considering that there may be administration-related changes in blood sugar control and appetite or eating behavior. Figure 3 shows the results of the survey. The means for the eating behavior profile before administration were similar between the improvement and non-improvement groups. In the former, the "external eating behavior", "emotional eating behavior", "sense of hunger", and "food preference" scores improved 3 months after the start of administration, whereas there were no profile changes after administration in the latter.
Figure 3: Questionnaire regarding eating behavior before and after treatment We performed survey regarding eating behavior by using "Questionnaire Regarding Eating Behavior" from the Japan Society for the Study of Obesity questionnaire, as described in detail in the text.
Discussion
The present study clearly demonstrated that liraglutide administration for 3 months decreased the mean HbA1c and body weight by approximately 0.6% and 1.9 kg, respectively. However, there were marked individual differences in the effects. Liraglutide reduced the blood sugar level in some patients, but increased it in others. Then, we tried to compare the clinical characteristics of the improvement group with those of the non-improvement group. In the former, the duration of disease was shorter, and the ability of insulin secretion was maintained favorably. In the natural history of type 2 diabetes, apoptosis of β cells occurs with the time course, reducing insulin secretion[5]. It may be very important to introduce liraglutide therapy early after onset, when the β cell function is maintained. The present data also demonstrated that furthermore long time treatment with liraglutide, such as 6 or 12 month - treatment showed the same effect on improving HbA1C as that observed at 3 - month treatment. Thus, it is suggested that we can speculate the long - term useful effect of liraglutide on controlling hyperglycemia by evaluating whether or not liraglutide can reduce hyperglycemia within 3 months.
In this study, CPR was measured before breakfast. However, several studies have indicated that the efficacy of liraglutide can be predicted based on the insulin secretory response on dietary and glucagon - loading tests[6]. In the future, a more detailed study involving a large number of patients should be conducted to clarify the most accurate index to predict the effects of liraglutide and establish a cut - off value.
GLP - 1 analogue preparations, including liraglutide, are known to promote insulin secretion in the pancreas, suppress glucagon secretion, delay gastric emptying, and reduce the appetite through central actions[7]. In this study, we evaluated the eating behavior pattern of the subjects using a questionnaire. In the improvement group, the eating behavior pattern improved in many aspects. However, there were no liraglutide administration - related changes in eating behavior in the non - improvement group. When liraglutide administration improves the HbA1c value, not only a decrease in the blood sugar level related to the enhancement of insulin secretion but also an improvement in a patient's eating behavior may be involved in the mechanism. Thus, liraglutide was effective in patients with a short duration of disease in whom intrinsic insulin secretion, which was determined as CPR index, was maintained, and a decrease in the blood sugar level was closely associated with an improvement in a patient's eating behavior.
Conclusion
A study on switching from another drug to liraglutide therapy was conducted in patients with type 2 diabetes who had been treated at the outpatient clinic. After 3 - month administration, the results showed that liraglutide was effective in patients with a short duration of disease in whom intrinsic insulin secretion was well maintained, and that a decrease in the blood sugar level was closely associated with an improvement in a patient's eating behavior.
Acknowledgements
This research was supported by research funds to promote the hospital functions of Japan Labour Health and Welfare Organization.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1. Rigato, M., Fadini, G.P. Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. ( 2014) Diabetes Metab Syndr Obes 7: 107-120.
- 2. Nauck, M., Frid, A., Hermansen, K., et al. Efficacy and safety comparison of liraglutide, glimepiride, and lacebo, all in combination mono therapy for type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. (2009) Diabetes Care 32: 84-90.<
- 3. Inoue, K., Maeda, N., Kashine, S., et al. Short-term effects of liraglutide on visceral fat adiposity, appetite, and food preference: a pilot study of obese Japanese patients with type 2 diabetes. (2011) Cardiovasc Diabetol 10: 109-116.
- 4. Fujishima, Y., Maeda, N., Inoue, K., et al. Efficacy of liraglutide, a glucagon-like peptide-1 (GLP-1) analogue, on body weight, eating behavior, and glycemic control, in Japanese obese type 2 diabetes.(2012) Cardiovasc Diabetol 11: 107-114.
- 5. Rudenski, A.S., Hadden, D.R., Atkinson, A.B., et al. Natural history of pancreatic islet β - cell function in type 2 diabetes mellitus studies over six years by homeostasis model assessment.(1988) Diabetes Med 5: 36-41.
- 6. Thong, K.Y., McDonald, T.J., Hattersley, A.T., et al. The association between postprandial urinary C-peptide creatinine ratio and the treatment response to liraglutide: a multi-centre observational study. (2014) Diabet Med 31: 403-411.
- 7. Seufert, J., Gallwitz, B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. (2014) Diabetes Obes Metab 16: 673-88.