Functional Roles of Shear Stress in Vascular Endothelial Cells
Baohua Huang1, Fang Yang1, Wei Shu2, Zhenfeng Chen1, Ming Chen1*
Affiliation
- 1State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmacy, Guangxi Normal University, China
- 2Department of Cell Biology and Genetics, Guangxi Medical University, China
Corresponding Author
Ming Chen, State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, School of Chemistry and Pharmacy, Guangxi Normal University, Guilin, China, E-mail: chenmingprotein@163.com
Citation
Chen, M., et al. Functional Roles of Shear Stress in Vascular Endothelial Cells. (2017) Cell Immunol Serum Biol 3(1): 64- 67.
Copy rights
© 2016 Chen, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Shear stress; Endothelial cells; Gene expression; Cell morphology
Abstract
Endothelial cells play remarkable roles in regulating vascular function in health and disease. Meanwhile, shear stress, which is created due to the friction of the flowing blood on the endothelium of the arterial wall, is critical for vascular homeostasis. Here, the shear stress patterns and its effect on gene expression and vascular function are reviewed.
Introduction
Endothelial cells (ECs), which line the luminal surface of blood vessel, play remarkable roles in regulating vascular function in health and disease[1]. Most of the previous studies reveal that ECs control vascular tone, permeability, inflammation, and even the growth and regression of blood vessels[2-5]. Because ECs are mechanosensitive to both shear stress and circumferential strain from blood flow and blood pressure within the vascular system, it has been confirmed that ECs are essential to vascular homeostasis[1-5]. Specifically, ECs are known to be dysfunctional under pathology conditions, such as diabetes and atherosclerosis[6,7]. Therefore, endothelial dysfunction is often a result of altered vascular morphology and is a hallmark of many pathological and disease states, such as atherosclerosis, diabetes, hypertension and inflammation. It is well known that ECs are continuously exposed to shear stress, which is created by the blood flow over the luminal surface of blood vessel[1,8]. In this regards, the conception that shear stress can modulate EC function by activating the mechanosensors, intracellular signaling, gene expression, cell morphology and structural remodeling, has been pointed out and reviewed by others[9-11]. The aim of this short review is to provide a brief summary of the current knowledge with a focus on the shear stress and its functional roles in endothelial cell.
Shear stress and blood flow patterns
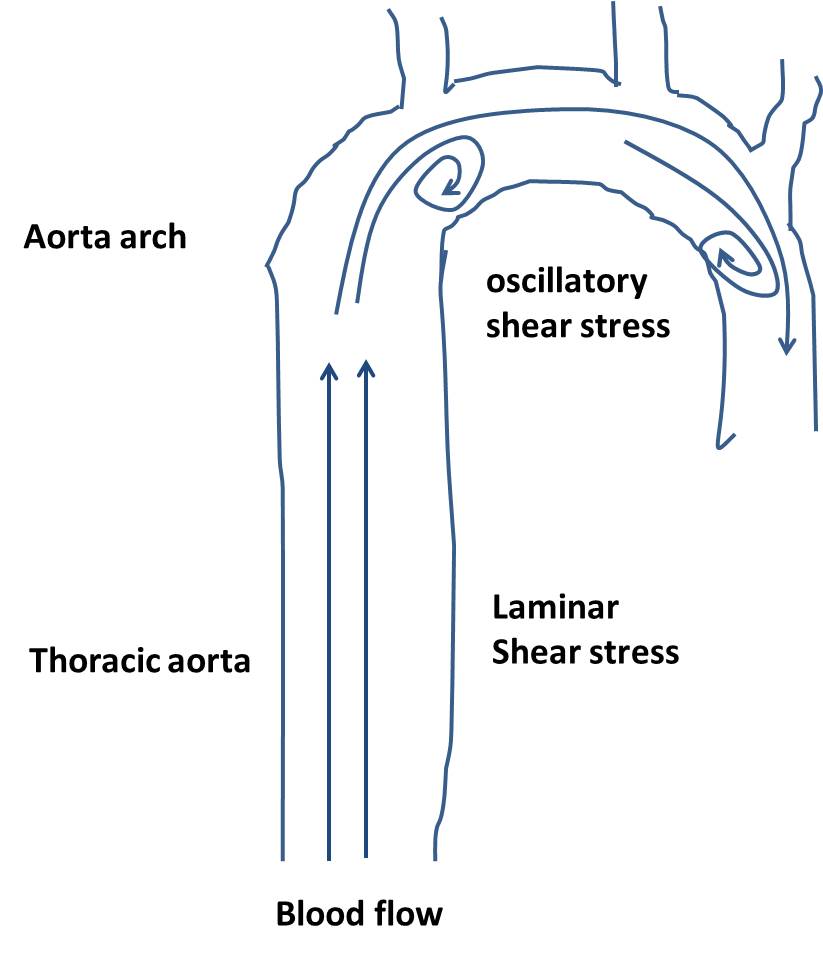
Shear stress is the force per unit area created due to the friction of the flowing blood on the endothelium of the arterial wall. In general, blood flow may be divided into either laminar or oscillatory shear flow[12]. The former is characterized by smooth streamlined flow, and the latter is characterized by areas of flow reversal[12,13]. Interestingly, the type of shear stress varies from region to region within the vasculature. For example, laminar shear stress is usually found in straight arterial regions, such as thoracic aorta and abdominal aorta area. However, oscillatory shear stress is found in branched or curved regions, such as aorta arch area, in which atherosclerosis occurred frequently (Figure 1)[14,15].
Shear stress and vascular function
Shear stress regulates gene expression in ECs: In vivo, ECs are exposed to shear stress caused by blood flow over the endothelial layer of blood vessels, which is a part of their normal physiological state[16]. In straight arterial regions with laminar shear stress flow, various athero-protective genes (such as KLF2) are highly expressed. However, several pro-atherogenic genes (such as ICAM-1, VCAM-1, MCP-1 and E-selectin) are significantly decreased, thereby leading to stability and quiescence of the ECs[17]. In contrast, in regions with low and oscillatory flow, the athero-protective genes are suppressed, while the pro-atherogenic genes are highly expressed. As a result, the endothelium develops different phenotypes to contribute the site-specific susceptibility for the initiation and progression of atherosclerosis. Shear stress also has been shown to involve in regulating the inflammatory processes through modification of endothelial gene expression[18]. Using microarray assay of endothelium under conditions of laminar or oscillatory shear stress, many groups have shown that oscillatory shear stress can result in pro-inflammatory gene expression in the vascular wall. However, laminar shear stress promotes an anti-inflammatory gene expression[10]. Up to date, the molecular mechanisms responsible for transducing shear stress and stretch into gene transcriptional changes are poorly understood.
Shear stress regulates eNOS activity in ECs: Nitric oxide (NO) plays a key role in endothelial function and vascular homeostasis. It has been reported that shear stress could regulate NO production by phosphorylating endothelial nitric oxide synthase (eNOS) at Ser 1177, Ser 633, Ser 635 and Tyr 657[19-22]. Among them, Ser 1177, Ser 633, and Ser 635 are active sites, however, Tyr 657 is inhibitory site. For example, laminar shear stress promotes activity of Akt, which in turn phosphorylates eNOS at Ser1177, and resulting in eNOS activation and NO production. Boo, et al. reported that shear stress stimulates phosphorylation of eNOS at Ser635 by a PKA-dependent mechanism[23]. Chen, et al. also reported the laminar shear stress increases the eNOS-SIRT1association and eNOS deacetylation, as well as enhances the phosphorylation of eNOS at Ser-633 and Ser-1177 via AMP-activated protein kinase (AMPK) pathway(1).
Shear stress regulates EC proliferation: Keeping ECs in quiescence state is required for vascular heath and homeostasis. Evidence from in vivo animal model show that increased EC proliferation is an early event of atherogenesis[24]. In vitro studies also show that laminar shear stress prevents ECs from entering S phase, resulting in most of cells are arrested in the G0/G1 phase. In contrast, cells exposed to oscillatory shear stress have accelerated turnover rate with enhanced G0/G1–S transition[25].
Shear stress regulates EC migration: EC migration is important in many biological processes, including angiogenesis and development of vascular diseases. For example, migration of ECs is an essential part of the development of wound healing and atherosclerosis. Simmers, et al. reported that arterial shear stress regulates endothelial cell-directed migration and polarity in confluent monolayers[26]. Sprague, et al. shown that EC migration in wound healing is significantly enhanced by laminar shear stress by using the flow channel in vitro[27], whereas oscillatory flow has less effect on wound healing. The mechanisms on this process are complex, it is reported that the shear-induced lamellipodia protrusion and focal adhesions remodeling may play key role in EC migration[28].
Taken together, shear stress can regulate the function of ECs by modulating the gene expression and phenotype of vascular ECs. Laminar shear stress activates signal transduction pathways and gene expression in ECs to suppress cell proliferation, inflammation, and atherosclerosis. In contrast, oscillating shear stress promotes atheroprone phenotype of ECs and increases EC proliferation, inflammation, leukocyte adhesion, thus contributing to atherogenesis[8,12,14,15,18,29].
Shear stress regulates endothelial cell morphology
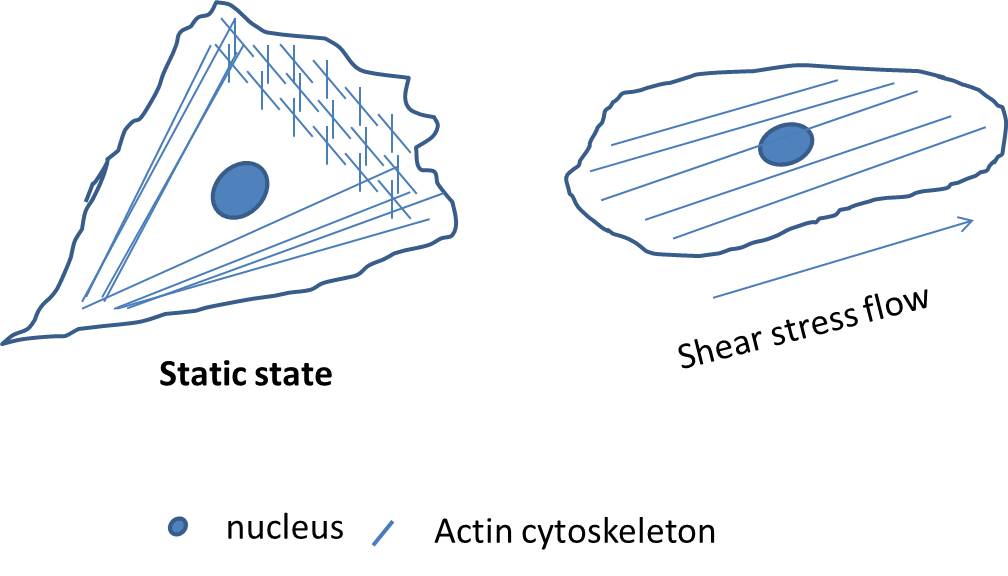
Shear stress regulates cytoskeleton re-organization: Laminar shear stress alters the endothelial cells shape has been observed for many decades[13,30]. When look at the cell morphology it could be found that endothelial cells are elongated, aligned in areas of high laminar shear stress[13]. However, endothelial cells are randomly orientated at areas of low laminar or oscillatory shear stress. Recently, the conception that shear stress has effect on the endothelial cytoskeleton, especially the act in and the microtubule cytoskeletons, has been proved by many groups[28-31]. Act in filaments are arranged both centrally and peripherally in endothelial cells to form stress fibers. In normal cells, the morphology of ECs were randomly aligned and uniformly polygonal, whereas ECs under laminar shear stress condition were elongated from the typical cobblestone pattern to uniformly fusi form aligned in the direction similar in appearance to the cells grown under laminar shear stress (Figure 2). In this condition, the stress fibers become both thicker and longer, and run mainly in the longitudinal direction of the cell[32]. The mechanism how shear stress regulates the act in filaments re-organization, however, remains undefined.
Shear stress regulates stress fiber anchoring to plasma membrane: Stress fibers are associated with plasma membrane at the leading edge of cell, and this association plays an important role in cell-cell junction formation and cell mobility, and thus is critical for endothelial cell migration and angiogenesis[33,34]. For example, stress fibers are found to link to the plasma membrane at cell junction or focal adhesion sites where they serve to promote strong attachment to the substratum. Stress fibers are reported to associate with and terminate at VE-cadherin-based cell–cell contacts[35]. It was reported that F-actin cytoskeleton directly or indirectly associates with focal adhesion complex through the F-actin binding tandem cortactin repeatsand the N-terminal acidic domain that interacts with the actin-related protein (Arp) 2/3 complex[36]. However, the localization and distribution of stress fibers and focal adhesions seem to be affected by the power of the local fluid shear stress force[37].
Conclusions and Perspectives
Shear stress plays critical roles in vascular system by regulating EC’s functions via activity of signaling pathway, and thus, leading to genes expressions and functional responses. Many of these responses are just beginning to be understood, although some cues had been discovery by many colleagues, the mechanisms how cells response to shear stress and the details need to be further explored. On the other hand, laminar shear stress has been reported to change EC morphology and keep the cells in heath state, and indicated its athero-protective function. However, these genes, which are responsible for this process, are still unclear. So, we think discovery and investigations of these genes are likely to provide some surprises, and some works are ongoing in our lab at Guangxi Normal University. The results of such investigations will advance our understanding of the physiological and pathological processes in vascular.
Acknowledge:
This work was supported by the grants from the Chinese Natural Science Foundation (No.31660242 to M.C.), the Guangxi Natural Science Foundation (No. 2016GXNSFCB380001 to M.C.) and the Key State Laboratory Talent Project (No.CMEMR2016-A01to M.C.)
Competing Interest:
The authors declare that they have no conflicts of interest with the contents of this article.
Abbreviations:: ECs: Endothelial Cells; ENOS: Endothelial Nitric Oxide Synthase; NO: Nitric Oxide; KLF2: Kruppel - Like Factor 2; ICAM-1: Intercellular Cell Adhesion Molecule-1; VCAM-1: Vascular Cell Adhesion Molecule-1; MCP-1: Monocyte Chemotactic Protein-1; AMPK: Activated Protein Kinase; SIRT1: Sirtuin 1
References
- 1. Chen, Z., Peng, I.C., Cui, X., et al. Shear stress, SIRT1, and vascular homeostasis. (2010) Proc Natl Acad Sci USA: 107(22): 10268-10273.
- 2. Loga, F., Domes, K., Freichel, M., et al. The role of cGMP/cGKI signalling and Trpc channels in regulation of vascular tone. (2013) Cardiovasc Res 100(2): 280-287.
- 3. Gavard, J., Patel, V., Gutkind, J.S. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. (2008) Dev Cell 14(1):25-36.
- 4. Okutani, D., Lodyga, M., Han, B., et al. Src protein tyrosine kinase family and acute inflammatory responses. (2006) Am J Physiol Lung Cell Mol Physiol 291(2): L129-L141.
- 5. Bazzoni, G., Dejana, E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. (2004) Physiol Rev 84(3): 869-901.
- 6. Zernecke, A., Weber, C. Chemokines in the vascular inflammatory response of atherosclerosis. (2010) Cardiovasc Res 86(2): 192-201.
- 7. Advani, A., Connelly, K.A., Advani, S.L., et al. Role of the eNOS-NO system in regulating the antiproteinuric effects of VEGF receptor 2 inhibition in diabetes. (2013) Biomed Res Int 2013:201475.
- 8. Li, Y.S., Haga, J.H., Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. (2005) J Biomech 38(10): 1949-1971.
- 9. Houston, P., Dickson, M.C., Ludbrook, V., et al. Fluid shear stress induction of the tissue factor promoter in vitro and in vivo is mediated by Egr-1. (1999) Arterioscler Thromb Vasc Biol 9(2): 281-289.
- 10. Ohura, N., Yamamoto, K., Ichioka, S., et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. (2003) J Atheroscler Thromb 10(5): 304-313.
- 11. Bond, A.R., Iftikhar, S., Bharath, A.A., et al. Morphological evidence for a change in the pattern of aortic wall shear stress with age. (2011) Arterioscler Thromb Vasc Biol 31(3): 543-550.
- 12. Dardik, A., Chen, L., Frattini, J., et al. Differential effects of orbital and laminar shear stress on endothelial cells. (2005) J Vasc Surg 41(5): 869-880.
- 13. Reidy, M.A., Langille, B.L. The effect of local blood flow patterns on endothelial cell morphology. (1980) Exp Mol Pathol 32(3): 276-289.
- 14. Cunningham, K.S., Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. (200) Lab Invest 85(1): 9-23.
- 15. Asakura, T., Karino, T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. (1990) Circ Res 66(4): 1045-66.
- 16. Pan, S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. (2009) Antioxid Redox Signal 11(7): 1669-1682.
- 17. Morigi, M., Zoja, C., Figliuzzi, M., et al. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. (1995) Blood 85(7):1696-1703.
- 18. Zakkar, M., Angelini, G.D., Emanueli, C. Regulation of Vascular Endothelium Inflammatory Signalling by Shear Stress. (2016) Curr Vasc Pharmacol 14(2): 181-186.
- 19. Boo, Y.C., Hwang, J., Sykes, M., et al. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. (2002) Am J Physiol Heart Circ Physiol 283(5): H1819-H1828.
- 20. Zhang, Y., Liao, B., Li, M., et al. Shear stress regulates endothelial cell function through SRB1-eNOS signaling pathway. (2016) Cardiovasc Ther 34(5): 308-313.
- 21. Li, B., Zhang, J., Wang, Z., et al. Ivabradine Prevents Low Shear Stress Induced Endothelial Inflammation and Oxidative Stress via mTOR/eNOS Pathway. (2016) PLoS One 11(2): e0149694.
- 22. Huang, B., Chen, C.T., Chen, C.S., et al. Laminar shear flow increases hydrogen sulfide and activates a nitric oxide producing signaling cascade in endothelial cells. (2015) Biochem Biophys Res Commun 464(4): 1254-1259.
- 23. Boo, Y.C., Sorescu, G.P., Bauer, P.M., et al. Endothelial NO synthase phosphorylated at SER635 produces NO without requiring intracellular calcium increase. (2003) Free Radic Biol Med 35(7): 729-741.
- 24. Zou, T., Liu, W.J., Li, S.D., et al. TRB3 mediates homocysteine-induced inhibition of endothelial cell proliferation. (2011) J Cell Physiol 226(11): 2782-2789.
- 25. Lin, K., Hsu, P.P., Chen, B.P., et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. (2000) Proc Nati Acad Sci U S A 97(17): 9385-9389.
- 26. Simmers, M.B., Pryor, A.W., Blackman, B.R. Arterial shear stress regulates endothelial cell-directed migration, polarity, and morphology in confluent monolayers. (2007) Am J Physiol Heart Circ Physiol 293(3): H1937-H1946.
- 27. Sprague, E.A., Luo, J., Palmaz, J.C. Human aortic endothelial cell migration onto stent surfaces under static and flow conditions. (1997) J Vasc Interv Radiol 8(1 Pt 1): 83-92.
- 28. Hu, Y.L., Li, S., Miao, H., et al. Roles of microtubule dynamics and small GTPase Rac in endothelial cell migration and lamellipodium formation under flow. (2002) J Vasc Res 39(6): 465-76.
- 29. Warboys, C.M., Eric Berson, R., Mann, G.E., etal. Acute and chronic exposure to shear stress have opposite effects on endothelial permeability to macromolecules. (2010) Am J Physiol Heart Circ Physiol 298(6): H1850-H1856.
- 30. Barbee, K.A., Davies, P.F., Lal, R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. (1994) Circ Res 74(1): 163-171.
- 31. Slee, J.B., Lowe-Krentz, L.J. Actin realignment and cofilin regulation are essential for barrier integrity during shear stress. (2013) J Cell Biochem 114(4): 782-795.
- 32. Malek, A.M., Izumo, S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. (1996) J Cell Sci 109( Pt 4): 713-726.
- 33. Tojkander, S., Gateva, G., Lappalainen, P. Actin stress fibers--assembly, dynamics and biological roles. (2012) J Cell Sci 125(Pt 8): 1855-1864.
- 34. Pellegrin, S., Mellor, H. Actin stress fibres. (2007) J Cell Sci 120(Pt 20): 3491-3499.
- 35. Millan, J., Cain, R.J., Reglero-Real, N., et al. Adherens junctions connect stress fibres between adjacent endothelial cells. (2010) BMC Biol 8:11.
- 36. Abu Taha, A., Taha, M., Seebach, J., et al. ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. (2014) Mol Biol Cell 25(2): 245-256.
- 37. Katoh, K., Kano, Y., Ookawara, S. Morphological differences between guinea pig aortic and venous endothelial cells in situ. (2007) Cell Biol Int 31(6): 554-564.