Genetic Control of Enamel Development
Amer Sehic1*, Qalb-E-Saleem Khan2, Cuong Khuu1, Minou Nirvani3,Tor Paaske Utheim1,4
Affiliation
- 1Department of Oral Biology, University of Oslo, Norway
- 2Department of Medical Biology, Faculty of Health Sciences, University of Tromsø, Norway
- 3Dental Office Majorstua, Oslo, Norway
- 4Department of Medical Biochemistry, Oslo University Hospital, Norway
Corresponding Author
Amer Sehic, Department of Oral Biology, University of Oslo, Oslo, Norway. E-mail: amer.sehic@odont.uio.no
Citation
Sehic, A. Genetic Control of Enamel Development. (2015) J Dent & Oral Care 1(3): 1- 3.
Copy rights
© 2015 Sehic, A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Dental enamel; Ameloblasts; Amelogenesis; Stem cells; Mouse incisor tooth
Abstract
Dental enamel is the only mineralized tissue of epithelial origin in mammals. The exceptional structural complexity and physical properties of tooth enamel seem to be dependent upon the properties of the protein matrix precursor. Proteins involved in enamel biosynthesis guide hydroxyapatite mineral formation, making the tooth enamel the hardest tissue in the vertebrate body. In dentistry, due to inflammatory or traumatic events, dental tissues including enamel may suffer from loss of their function. The regenerative capability of dental enamel is fundamentally limited due to apoptosis of ameloblasts after tissue maturation and tooth eruption. Today, replacing the lost enamel in dental practice relies on restorative materials, such as polymers, metals and ceramics, which frequently fail due to poor adhesion or cracking. Therefore, further understanding of events during enamel formation and accordingly biomimetic replacement for dental enamel is required.
Introduction
Teeth share similar developmental pathways with other organs, such as kidneys, lungs, hair, skin and salivary glands. In tooth development, morphogenesis and differentiation of ameloblasts, odontoblasts and cementoblasts are regulated by a succession of epithelial-mesenchymal interactions that leads to the formation and mineralization of the dental hard tissues. Dental enamel is the only mineralized tissue of epithelial origin in mammals and its structure is exceptional because of its high mineral content[1]. The formation of enamel matrix and subsequent mineralization are a result of expression of tissue-specific genes in differentiated enamel forming cells, ameloblasts. Enamel is constituted of highly organized, tightly packed hydroxyapatite crystallites, Ca10(PO4)6(OH)2, making up about 95% of its weight and 87% of its volume[1]. Mature dental enamel contains less than 1% organic matter, whereas other mineralized tissues constitutes of about 20% organic material. Hydroxyapatite crystallites in dental enamel contain more than one thousand times the volume of corresponding crystals in dentin, cementum and bone. Enamel crystals are highly oriented and organized by differential organization into a pattern of prisms and interprism. Enamel prisms, each produced by single ameloblast, are extremely long relative to their thickness and extend from the enamel-dentin junction toward the surface of the tooth. The spatial arrangement of highly mineralized enamel crystals and their structural complexity give dental enamel its unique physical properties, making it the hardest tissue in the vertebrate body. Since the structure of mature dental enamel is a result of events that took place during enamel formation, the disturbances in the function of ameloblasts during enamel formation often lead to permanent defects in enamel such as hypo-mineralization and/or hypoplasia.
In general, the scientific research in medical field aims to provide materials and techniques in order to restore the loss of damaged tissues. In order to repair and/ or replace the defective tissues or organs, it is of outmost importance to understand in detail the developmental events of specific tissues. In dentistry, due to inflammatory or traumatic events, dental tissues may suffer from loss of their function. It is therefore an expanding requirement for predictable therapeutic procedures and routines that make it able to regenerate damaged and lost tissues. Today we know that stem cells play significant roles in organogenesis and tissue repair. Already in 1768, Fougeroux, a French naturalist, demonstrated that the teeth of a rabbit, unlike those of humans, grow continuously[2]. About 40 years later Oudet experimentally confirmed this interesting finding by cutting off rabbit incisors at the gingival level and showing that these teeth indeed regenerated[3]. Findings by Fougeroux and Oudet made the essential basis for the discovery two centuries later that the continuous growth of incisors in rabbits and rodents is provided by stem cells that are situated in the proximal end of the incisor tooth and produce all essential cell types throughout the animal’s life.
Dental enamel formation is a significantly controlled genetic process. Conclusive evidence has shown that the shape, size, and even caries susceptibility of dental enamel may be inheritable, and can be passed from parent to offspring. The studies of inheritance patterns in twins, families, and animal breeding were compatible with a genetic fraction for susceptibility to dental caries[4]. Inheritable genetic mechanisms involved in formation of dental enamel result in a phenotype that is more susceptible to demineralization, which may explain increased susceptibility to dental caries. This is in accordance with recent investigation that has identified genetic varieties in genes essential for enamel formation that are associated with increased levels of caries experience. Mutation in one of these genes, X-linked amelogenin (AMELX), results in a defect in tooth enamel called amelogenesis imperfecta. It has therefore been hypothesized that differential expression of AMELX may result in structural and/or compositional changes in enamel that could make persons more or less susceptible to acid demineralization and caries progression[4]. Other genetic disorders may be associated with enamel malformations that range from localized minor defects to total absence of tooth enamel. It is noticeable that formation of dental enamel is highly specialized, and that proteins primarily involved in enamel biosynthesis are specific for it. Consequently, imperfections in the genes coding for enamel proteins mainly cause enamel malformations without affecting other organs or parts of the body.
In contrast to humans, mice, which are a fundamental and commonly used model for research in odontogenesis, have a highly specialized dentition. The permanently growing adult mouse incisor has been considered as a tempting model system for the study of adult stem cells over the past several years[5], and most of the current knowledge about the regulation of these cells is based on the studies on the mouse incisors[5-7]. Stem cells are present in several different organs and are essential for normal development and homeostasis as well as repair of tissue damage. Investigations using genetically modifiable mouse, together with other experimental approaches such as explant cultures, have significantly improved our understanding of the underlying mechanism and genetic signaling networks that are implicated in the development and the renewal of the rodent incisor.
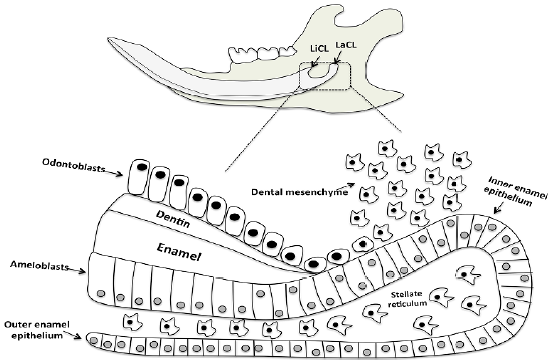
There is a continuous abrasion at incisal end of the rodent incisor, which is compensated by growth at its apical end[8,9]. Continuously growing rodent incisors exhibit all stages of tooth development along their inciso-apical axis, and this characteristic makes them suitable model for the study of enamel formation. For several years the rat incisor has been extensively used for this purpose, and its normal enamel structure is well characterized[10]. Today we know that this permanent growth of the incisor tooth is due to the presence of a stem cell niche presented within the cervical loops at the apical part of the tooth (Figure 1). The lingual and labial cervical loops are asymmetric in size, with the labial cervical loop being significantly larger due to different functional demands. Only cells within the labial cervical loop are able to differentiate into enamel-producing ameloblasts, stellate reticulum, stratum intermedium, and outer dental epithelium, and thereby produce enamel on the labial aspect of the tooth[11,12]. However, the lingual cervical loop is considerably smaller and its progeny do not give rise to ameloblasts (Figure 1). The cells within the lingual cervical loop are generated into the inner dental epithelium which supervise terminal differentiation of mesenchymal cells into odontoblasts[13]. Therefore, the lingual cervical loop serves as a root-analogous compartment anchoring the incisor into the jaw[14].
Figure 1: Schematics of mouse mandibular jaw with molar teeth and continuously growing incisor tooth. Higher magnification of the labial cervical loop; a stem cell progeny in the epithelial stem cell niche of the continuously growing incisor tooth is shown. The stem cell compartment is presented at the apical part (proximal end) of the tooth. The stem cells continuously supply ameloblasts and odontoblasts, cells that are responsible for replacing the worn enamel and dentin, respectively. The incisor epithelial stem cells are located in the outer enamel epithelium and stellate reticulum of the labial cervical loop. These cells give rise to epithelial cells in inner enamel epithelium that undergo massive proliferation and differentiate into ameloblasts, while the stem cells from dental mesenchyme generate odontoblasts.
LiCL: lingual cervical loop, LaCL: labial cervical loop.
In humans, a great variety exists with regard to the shape and number of teeth. The temporary teeth erupt at around six months of age and are fully replaced by permanent teeth by the early teen years. Once the tooth erupts into the oral cavity, the dental epithelium is lost, and consequently the regenerative capability of dental enamel is fundamentally limited. Therefore, further understanding of events during enamel formation and accordingly biomimetic replacement for dental enamel is required. Synthetic approaches are needed in order to promote enamel formation as well as aim to repair damage and increase the longevity of teeth. When the underlying molecular mechanisms of dental enamel formation become established, it is likely that biomimetic enamel will become available as a restorative material in dentistry. Currently, the recombinant enamel proteins are being investigated for their effects on hydroxyapatite crystal growth, but significantly improved understandings are needed before tooth enamel can be synthesized in the lab and used in clinic. Hopefully, stem cell research together with increasing understanding of odontogenesis may guide us in the future to the growth of teeth in culture for use as dental implants. At least, this is theoretically possible, and should be prioritized area in research for the coming years.
References
- 1. Simmer, J.P., Hu, J.C. Dental enamel formation and its impact on clinical dentistry. (2001) JDent Educ 65(9): 896- 905.
- 2. Fougeroux de Bondaroy, A.D. Observations anatomiques. (1768) Hist Acad Roy Sci Paris 47-48.
- 3. Oudet, J. Experiments continued l`accroissement and reproduction of teeth in rabbits. (1823) J Phisiol Expér Pathol.
- 4. Vieira, A.R., Gibson, C.W., Deeley, K., et al. Weaker dental enamel explains dental decay. (2015) PLoS One 10(4): e0124236.
- 5. Kuang-Hsien Hu, J., Mushegyan, V., Klein, O.D. On the cutting edge of organ renewal: Identification, regulation, and evolution of incisor stem cells. (2014) Genesis 52(2): 79- 92.
- 6. Bossu, M., Pacifici, A., Carbone, D., et al. Today prospects for tissue engineering therapeutic approach in dentistry. (2014) Scientific World Journal 9.
- 7. Xiao, L., Nasu, M. From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. (2014) Stem Cells Cloning 7: 89- 99.
- 8. Harada, H., Kettunen, P., Jung, H.S., et al. Localization of putative stem cells in dental epithelium and their association with Notch and FGF signaling. (1999) J Cell Biol 147(1): 105- 120.
- 9. Smith, C.E., Warshawsky, H. Histological and three dimensional organization of the odontogenic organ in the lower incisor of 100 gram rats. (1975) Am J Anat 142(4): 403- 429.
- 10. Warshawsky, H. A light and electron microscopic study of the nearly mature enamel of rat incisors. (1971) Anat Rec 169(3): 559- 583.
- 11. Sehic, A., Peterkova, R., Lesot, H., et al. Distribution and structure of the initial dental enamel formed in incisors of young wild-type and Tabby mice. (2009) Eur J Oral Sci 117(6): 644- 654.
- 12. Sehic, A., Risnes, S., Khan, Q.E.,et al. Gene expression and dental enamel structure in developing mouse incisor. (2010) Eur J Oral Sci 118(2): 118- 130.
- 13. Thesleff, I., Vaahtokari, A., Vainio, S. Molecular changes during determination and differentiation of the dental mesenchymal cell lineage. (1990) J BiolBuccale 18(3): 179- 188.
- 14. Tummers, M., Thesleff, I. Observations on continuously growing roots of the sloth and the K14-Eda transgenic mice indicate that epithelial stem cells can give rise to both the ameloblast and root epithelium cell lineage creating distinct tooth patterns. (2008) EvolDev 10(2): 187- 195.