Microalbuminuria in Diabetes
Khushbu Yadav
Affiliation
- 1Assistant Professor, Department of Biochemistry, Janaki Medical College Teaching Hospital, Janakpurdham, Nepal
- 2Medical Microbiologist and Lecturer, Krishna Medical Technical Research Center, Janakpurdham, Nepal
Corresponding Author
Satyam Prakash, Department of Biochemistry, Janaki Medical College Teaching Hospital, Tribhuvan University, Janakpur-45600, Nepal; Tel: +977-9844405444, E-mail: sprakashy2424@gmail.com
Citation
Prakash, S., Yadav, K. Microalbuminuria in Diabetes. (2017) Lett Health Biol Sci 2(1): 52- 60.
Copy rights
© 2017 Prakash, S. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Complications; Diabetes; Diabetic nephropathy; Microalbuminuria
Abstract
Diabetes has become the main public health challenge for the 21st century. Pronounced changes in the human environment and in human behavior and lifestyle, have accompanied globalization, and these have resulted in escalating rates of diabetes. Microalbuminuria is considered as a clinically important indicator of deteriorating renal function in diabetic patients. Microalbuminuria is the strong predictor of diabetic nephropathy, which is the main cause of mortality and morbidity in patients with diabetes mellitus. It is also characterized by increased prevalence of arterial hypertension, proliferative retinopathy, and peripheral neuropathy. Detection of microalbuminuria is an indication for initiation of appropriate therapy for the purpose of preventing the advance of progressive diabetic nephropathy. Diabetic kidney disease or nephropathy is the most common cause of end stage renal disease (ESRD) or kidney failure. One of the early markers of not only diabetic nephropathy, but also vascular disease in patients with diabetes, is the presence of microalbuminuria. The primary constituent of urinary protein in diabetic nephropathy is albumin. Consequently, quantification of urinary albumin excretion is central to any description of diabetic renal disease. Other renal diseases that occur with greater frequency in diabetic patients include asymptomatic bacteriuria, pyelonephritis, papillary necrosis, and radiocontrast induced renal failure. Primary prevention of diabetes is the ideal. In this concern, this review briefly highlights the features of diabetes, diabetic nephropathy and different perspectives of microalbuminuria in diabetes.
Introduction
Diabetes is now taking its place as one of the main threats to human health nowadays[1,2]. The past two decades have seen rapid increase in the number of people diagnosed with diabetes worldwide[3,4]. Diabetes is a chronic disorder of carbohydrate, fat and protein metabolism due to insulin deficiency and /or insulin resistance, evolving from interaction of variety of genetic and environmental factors. The characteristic feature of diabetics is hyperglycemia and it represents a heterogeneous group of disorders that have a common feature of hyperglycemia[5].
The chronic hyperglycemia in diabetes causes several abnormalities of the host immune system which is frequently associated with permanent and irreversible functional and structural changes in the cells of the body with long-term damage, dysfunction and failure of various organs especially the eyes, kidneys, nerves, heart and blood vessels[6,7]. Elevated blood glucose induces oxidative stress and changes in the cellular redox state. Nicotinamide adenine dinucleotide oxidase (NADPH) has been responsible for the formation of high levels of reactive oxygen species (ROS) in response to high glucose[8,9].
There is growing evidence to suggest that diabetes mellitus (DM) is heterogeneous in etiology, clinical presentation and susceptibility to complication and response to treatment. The spectrum is to so wide that diabetes is presently regard as a syndrome rather than disease entity. DM is the most frequent endocrine disease whose prevalence and incidence varies worldwide[10] with severe medical complications. The complications of diabetes are retinopathy, neuropathy, nephropathy, cardiovascular complications and stroke. The patients with DM have increased risk of infection due to their weakened immune system[11].
Diabetes is the most common cause of end stage renal disease (ESRD) and is a major risk factor for cardiovascular disease and blindness[12]. Diabetic nephropathy is a common consequence of prolonged DM which is characterized by a progressive increase in the excretion of albumin, an early and continuing rise in systemic blood pressure, and alters glomerular filtration rate, leading eventually to End Stage Renal Disease (ESRD). In addition to these, central abnormalities, diabetic patients with nephropathy can be distinguished from their normoalbuminuric peers by the presence of other metabolic and clinical abnormalities[13,17]. This complication is first manifested as an increase in Urinary Albumin Excretion (UAE) often called as microalbuminuria, a stage called incipient nephropathy which progresses to overt albuminuria and then to renal failure[14].
Microalbuminuria represents an abnormally elevated urine albumin level that cannot be detected with the use of a urinalysis dipstick. The presence of microalbuminuria predicts worsening of renal disease to overt diabetic nephropathy and an elevated risk of cardiovascular disease[15-18]. Up to 30% of people with newly diagnosed type 2 diabetes will already have abnormally high urine albumin levels; about 75% of these people will have microalbuminuria and about 25% will have diabetic nephropathy[19-23].
Microalbuminuria is considered as a clinically important indicator of deteriorating renal function in diabetic and hypertensive patients. In these patients; the microalbuminuria phase is followed by progressive increase in urinary protein excretion and declining glomerular filtration rate. With improved methodology, these low levels of albumin (20 - 200 μg/min, 30 - 300 mg/24 h or 20 - 200 mg/L) can now be measured. Detection of microalbuminuria in DM is important from a clinical standpoint because, once detected, it is an indication for initiation of appropriate therapy for the purpose of preventing the advance of progressive diabetic nephropathy. It is important to assess factors related to the development of microalbuminuria in people with DM in order to identify whether such changes are due to underlying renal pathology or may be related to functional changes[24]. In this article we review the diabetes and strategies for microalbuminuria screening in diabetics to prevent the progression of renal disease.
Diabetes
Diabetes is defined as metabolic disorder due to relative or absolute deficiency of insulin which is caused by a failure of glucose homeostasis. The term diabetes mellitus describes a metabolic disorder of multiple etiology, which is characterized by hyperglycemia [fasting plasma glucose, FPG > 7.0 mmol/L or 126 mg/dL), fasting is defined as no caloric intake for at least 8 hours or two hour post 75 gm oral glucose load plasma glucose > 11.1 mmol/L (200 mg/dL), on two or more occasions], with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action or both.
Classification
An international expert committee released a report with new recommendations for the classification and diagnosis of diabetes mellitus. These new recommendations were the result of more than two years of collaboration among experts from the American Diabetes Association (ADA) and the World Health Organization (WHO). Clinical classification of diabetes mellitus adopted by WHO (1985)[25] are shown in table 1.
Table 1: Classification of Diabetes.
| Class | Name |
|---|---|
| 1 | Diabetes mellitus (DM) |
| a. Insulin-dependent diabetes mellitus ( IDDM, Type 1 ) | |
| b. Non-insulin dependent diabetes mellitus (NIDDM, Type 2 ) | |
| c. Malnutrition-related diabetes mellitus (MRDM) | |
| d. Other types | |
| 2 | Impaired glucose tolerance (IGT) |
| 3 | Gestational diabetes mellitus (GDM) |
Diabetes mellitus (DM)
Insulin-dependent diabetes mellitus (IDDM, Type 1): Type 1 diabetes is primarily due to autoimmune-mediated destruction of pancreatic β-cell islets, resulting in absolute insulin deficiency. People with type 1 diabetes must take exogenous insulin for survival to prevent the development of ketoacidosis. It is the most common chronic disease of children among 10 - 14 years but occasionally occurs in adults[26-28].
Type 1 diabetes is usually characterized by the presence of autoantibodies against the signaling mechanism in the islet cells. Its onset is typically abrupt and is usually seen in individuals less than 30 years of age. In some subjects with this clinical form of diabetes, no evidence of an autoimmune is demonstrable and these are classified as Type 1 idiopathic which have neither an etiology nor a pathogenesis[26]. It is lethal unless promptly diagnosed and treated. This form of diabetes is immune-mediated in over 90% of cases and idiopathic in less than 10% cases. Type 1 diabetic is usually associated with ketosis in its untreated state. Exogenous insulin is therefore required to reverse the catabolic state, prevent ketosis, reduce the hyperglucagonemia, and reduce blood glucose[29].
Non-insulin dependent diabetes mellitus (NIDDM, Type 2): Type 2 diabetes includes the common major forms of diabetes which results from defects in insulin secretion, almost always with a major contribution from insulin resistance and much more common than Type 1[30]. It is often discovered by chance. It is typically gradual in onset and occurs mainly in the middle-aged and elderly, frequently mild, slow to ketosis and is compatible with long survival if given adequate treatment. About 90.0% of persons (life time risk is 5 -7 %) with diabetes mellitus have this type of DM and is highly associated with a family history of diabetes, older age, obesity and lack of exercise. These patients have elevated plasma insulin level but have down regulated insulin receptor[5,31,32]. It is more common in women with a history of gestational diabetes. The etiology of Type 2 diabetes mellitus is multi-factorial and probably genetically based, but also has strong behavioral components. Type 2 diabetes is characterized by insulin resistance and/or abnormal insulin secretion, either of which may predominate. Insulin resistance and hyperinsulinemia eventually lead to impaired glucose tolerance and hyperglycemia. People with type 2 diabetes are not dependent on exogenous insulin, but may require it for control of blood glucose levels if this is not achieved with diet alone or with oral hypoglycaemic agents[2,33,34].
Malnutrition-related diabetes mellitus (MRDM): Malnutrition in early infancy and childhood may result in partial failure of β-cell function. A number of chemical agents are known to be toxic to beta cells e.g., alloxan, streptozotocin, the rodenticide VALCOR, cyanide producing foods (e.g. cassava and certain beans)[35,36].
Other types: Other types of diabetes are occur due to secondary to pancreatic, hormonal,drug-induced , genetic and other abnormalities[37].
Impaired glucose tolerance (IGT): Impaired glucose tolerance (IGT) describes a state intermediate at risk group between diabetes mellitus and normality. It can only be defined by the oral glucose tolerance test[13].
Gestational diabetes mellitus (GDM): Gestational diabetes mellitus (GDM) is carbohydrate intolerance resulting in hyperglycemia of variable severity with onset or during pregnancy. The definition applies irrespective of whether or not insulin is used for treatment or the condition persists after pregnancy[38-40].
Etiology of Diabetes Mellitus: Several pathogenesis processes are involved in the development of diabetes. Diabetes results from deficient insulin secretion, decreased insulin action or both. Many processes can be involved ranging from
1. Autoimmune destruction of the β-cells of the pancreas with consequent insulin deficiency.
2. Incomplete understood abnormalities that result in resistance to insulin action.
3. Defects in the formation of insulin e.g. synthesis of an abnormal, biologically inactive insulin molecule.
4. Genetic factors are involved in both type 1 and type 2 mechanisms.
Risk factors of diabetes mellitus
Diabetes may occur at any age, sex that may be due to the genetic factors, obesity, sedentary life styles, diet, malnutrition and certain chemical reagents as well as social factors. Stress (Surgery, trauma) also associated with diabetes. High rates of diabetes have been associated with a number of social factors such as occupation, marital status, religion, economic status, education, urbanization, changes in life style etc[38,39].
Malnutrition: Malnutrition related diabetes affects large number of young people. In some countries (eg U.K.) overall male to female ratio is about equal. In South–East Asia, an excess of male diabetes has been observed.
Genetic factor: The genetic nature of diabetes is undisputed. It was showed that in identical twins that developed Type 2 diabetes mellitus, concordance was 90%, thus demonstrating a strong genetic component. In Type 1 diabetes mellitus, the concordance was only about 50% indicating that this type of diabetes is not totally a genetic entity.
Obesity: Obesity particularly central adiposity has long been accepted as a risk factor for Type 2 diabetes and the risk is related to both the duration and degree of obesity[41]. Evephart JE, 1992 The association has been repeatedly demonstrated in longitudinal studies in different populations, with a striking gradient of risk apparent with increasing level of basal metabolic index (BMI), adult weight gain, waist circumference or waist to hip ratio. Indeed waist circumference or waist to hip ratio (reflecting abdominal or visceral adiposity) are more powerful risk factor of subsequent risk of type 2 diabetes than BMI[42]. Central obesity is also an important risk factor of insulin resistance, the underlying abnormality in most cases of type 2 diabetes[43,44]. Offsprings of diabetic pregnancies including gestational diabetes are often large and heavy at birth, tend to develop obesity in childhood and are at high risk of developing type 2 diabetes at an early age. Those born to mothers after they have developed diabetes have a three-fold higher risk of developing diabetes than those born before[42].
Sedentary life style: Sedentary life style appears to be an important risk factor for the development of type 2 diabetes. Lack of exercise may alter the interaction between insulin and its receptors and subsequently lead to type 2 diabetic[40].
Diet: A high saturated fat intake has been associated with a higher risk of impaired glucose tolerance, and higher fasting glucose and insulin levels. Higher proportions of saturated fatty acids in serum lipid or muscle phospholipids have been associated with higher fasting insulin, lower insulin sensitivity and a higher risk of type 2 diabetes. Excessive intake of alcohol can increase the risk of diabetes by damaging the pancreas and liver.
Diagnostic criteria for Diabetes Mellitus
In the part, the commonest approach to diabetes screening was a preliminary, semi-quantitative test for glucose in a urine sample, followed by an oral glucose tolerance test for those found to have glycosuria. Previously recommended oral glucose tolerance test by the National Diabetes Data Group has been replaced with the recommendation that the diagnosis of diabetes mellitus be based on two fasting plasma glucose levels of 126 mg/dL (7.0 mmol./L) or higher. Other options for diagnosis include two-hour post prandial plasma glucose (2 hr PPG) readings of 200 mg/dL (11.1 mmol/L) or higher after a glucose load of 75 gm (the criterion recommended by WHO) or two casual glucose readings of 200 mg/dL (11.1 mmol/L) or higher. Fasting plasma glucose was selected as the primary diagnostic test because it predicts adverse outcomes (e.g. retinopathy) and is much more reproducible than the oral glucose tolerance test or the 2 hr PPG test and easier to perform in a clinical setting[25].
Criteria of defining as diabetes mellitus according to the 1998 WHO- fasting glucose concentration of at least 6.1 mmol/L (110 mg/ dl) or a two-hour post prandial glucose concentration of at least 10.0 mmol / dl (180 mg/ dl). Blood glucose levels above the normal level but below the criterion established for diabetes mellitus indicate impaired glucose homeostasis. Persons with fasting plasma glucose levels ranging from 110 to 126 mg/dl (6.1 to 7 mmol/L) are said to have impaired fasting glucose, while those with 2 hr PPG level between 140 mg /dL (7.7 mmol/L) and 200 mg/ dL (11.1 mmol/L) are said to have impaired glucose tolerance. Both impaired fasting glucose and impaired glucose tolerance are associated with an increased risk of developing type 2 diabetes mellitus[25].
Table 2: Diagnostic values for the oral glucose tolerance test[25].
| Parameters | Whole blood | Plasma | ||
|---|---|---|---|---|
| Diabetes mellitus | Venous | Capillary | Venous | Capillary |
| Fasting value | ≥ 120 | ≥ 120 | ≥ 120 | ≥ 120 |
| 2 hrs after glucose load | ≥ 180 | ≥ 200 | ≥ 200 | ≥ 200 |
| Impaired glucose tolerance | ||||
| Fasting value | 110-126 | 110-126 | 110-126 | 110-126 |
| 2 hrs after glucose load | 140-200 | 140-200 | 140-200 | 140-200 |
The glucose tolerance test gives many false positives results because the test itself can be stress inducing, causing epinephrine release. This hormone decreases the release of insulin from the β-cells, and thus impairs the response to a glucose load. As a result, the glucose tolerance test is usually used only in situation in which diagnosis is uncertain or as a test for gestational diabetes. For general population, a fasting blood glucose test is the more commonly used diagnostic tool. Broadly type 1 diabetes is differentiated from type 2 diabetes on the basis of ketone bodies tests especially acetone. Acetone test is generally positive in fasting urine sample of type 1 diabetes.
Complications associated with Diabetes Mellitus
Intensive treatment with insulin delays the onset and slows the progression of these long-term complications. For example, the incidence of retinopathy decreases as control of blood glucose improves and HbA1C levels decrease. The benefits of tight control of blood glucose outweight the increased risk of severe hypoglycemia. How hyperglycemia causes the chronic complications of diabetes is unclear[45].
Long term complications
• Diabetic retinopathy
• Heart disease
• Diabetic neuropathy
• Diabetic foot diseases
• Nephropathy
Short term complications
• Glycosuria
• Hyperglycemia
• Ketoacidosis
• Hypertriacylglycerolemia etc
Infectious complications: Diabetic patients are susceptible to various types of infection. Several aspects of immunity are altered in patients with diabetes. Poly-morphonuclear leucocytes function is depressed, particularly when acidosis present[4]. Leucocytes adherence, chemotaxis and phagocytosis may be affected. Impaired host defense mechanisms such as impaired wound healing, impaired granulocyte function, decreased cellular immunity, impaired complement function and decreased lymphokine response may be influenced by glycemic control. So the diabetic individuals are not only predisposed to infections but that infection also complicates the control of the diabetes[46].
Frequently encountered infections in diabetes: Patients with diabetes mellitus are more predisposed to infections. This predisposition is due to a combination of anginopathy, neuropathy and hyperglycemia[47].
Foot infections: Diabetic patients are particularly susceptible to foot inections. These infections are leading cause of limb loss in the United States and are responsible for the majority of hospitalizations of diabetic patients[48].
Respiratory infections: Diabetic patients may be more predisposed to lower respiratory tract infections[49].
Skin infections: Diabetic patients appear to have skin infections more often than their non-diabetic counterparts. Poor host defense mechanism and a general environment conducive to the bacterial growth combine to make diabetic patient more susceptible to skin infections due to bacteria and fungi[50].
Upper soft tissue infections: The incidence of hand upper extremity infections is higher among diabetic patient. Hyperglycemia may be an independent predictor of postoperative infectious complications in diabetic patients and suggest a target glucose concentration of less than 200 mg/dl to reduce the risk of postoperative infection[47].
H. pylori infection: A higher frequency of Helicobacter pylori infection among dyspeptic diabetic patients than among non-diabetic patients was reported[51].
Urinary tract infection: Urinary tract infection is common in diabetic patients due to decreased resistance to infection. Diabetes predisposes the patient to renal abscesses which increase the risk of UTI. Infections increase the risk of nephropathy, which may place the patient at greater risk for developing kidney failure [14,52]. Anatomic and functional abnormalities of the urinary tract are also associated with diabetes. Women with diabetes are at a particularly increased risk of urinary tract infection[51].
Diabetic Nephropathy: Diabetic nephropathy, also known as Kimmelstiel-Wilson syndrome or nodular diabetic glomerulosclerosis or intercapillary glomerulonephritis, is a clinical syndrome characterized by albuminuria (>300 mg/day or >200 mcg/min) confirmed on at least two occasions 3 - 6 months apart, permanent and irreversible decrease in glomerular filtration rate (GFR)[53] and arterial hypertension[54]. The syndrome was first described by a British physician Clifford Wilson (1906- 1997) and American physician Paul Kimmelstiel (1900 - 1970) in 1936[55]. Diabetic nephropathy is a chronic complication of both type 1 DM(beta cell destruction – absolute lack of insulin) and type 2 DM (insulin resistance and/or decreased secretion of insulin).
Stages of Diabetic Nephropathy
There are five stages in the development of diabetic nephropathy. i.e.
Stage I - Hypertrophic hyper filtration: In this stage, GFR is either normal or increased. Stage I lasts approximately five years from the onset of the disease. The size of the kidneys is increased by approximately 20% and renal plasma flow is increased by 10% - 15%, while albuminuria and blood pressure remain within the normal range[56,57].
Stage II - Quiet stage: This stage starts approximately two years after the onset of the disease and is characterized by kidney damage with basement membrane thickening and mesangial proliferation. There are still no clinical signs of the disease. GFR returns to normal values. Many patients remain in this stage until the end of their life.
Stage III - Microalbuminuria stage: This stage is also called initial nephropathy. This is the first clinically detectable sign of glomerular damage. It usually occurs five to ten years after the onset of the disease. Blood pressure may be increased or normal. Approximately 40% of patients reach this stage[56,58].
Stage IV - Chronic kidney failure stage: Chronic kidney failure (CKF) is the irreversible stage. Proteinuria develops (albumin > 300 mg/dU), GFR decreases below 60 mL/min/1.73 m², and blood pressure increases above normal values.
Stage V - Terminal kidney failure stage (TKF) (GFR < 15 mL/min/1.73 m²): Approximately 50% of the patients with TKF require kidney replacement therapy (peritoneal dialysis, hemodialysis, kidney transplantation)[59]. In the initial stages of diabetic nephropathy, increased kidney size and changed Doppler indicators may be the early morphological signs of renal damage, while proteinuria and GFR are the best indicators of the degree of the damage[60].
Microalbuminuria
Diabetic micro-albuminuria is defined as the clinical diabetes with the diagnosed microalbumin in urine. All patients with type 1 or type 2 diabetes should be annually screened for micro-albuminuria. If a positive result for it is obtained in a type 1 diabetic, the patient’s treatment should be intensified to diminish urinary albumin excretion and to halt further deterioration of renal function[61-63]. In type 2 diabetics, microalbuminuria is a significant indicator of increased cardiovascular risk. The level of micro-albuminuria should be measured every two to three months until a plateau is reached. In type-2 diabetes, increased urinary albumin excretion is a strong independent predictor of progressive renal disease, atherosclerotic disease, cardiovascular mortality and overall mortality. Conversely, insulin resistance has been suggested to predict an elevation in urinary albumin excretion and to precede microalbuminuria[56].
Albumin is normally found in the blood and filtered by the kidneys. When the kidneys are working properly, albumin is not present in the urine. However, when the kidneys are damaged, small amount of albumin leak into the urine. This condition is called micro-albuminuria[43]. Micro-albuminuria is defined as the condition in which albumin is excreted (24 hr urine or time day collection) at a rate between 20 and 200 μg/min or 30 – 300 mg/24 hr. A normal albumin excretion rate ( AER) should amount to 2 to 20 μg /min or 3 to 30 mg/24 hr ,while a clinical albuminuria or micro-albuminuria is present at an excretion rate above 200 μg/min or >300 mg/24 hr[64]. Definition criteria of micro-albuminuria is given in Table 3.
Table 3: Definition of micro-albuminuria[59].
| Nature of Urine sample | Criteria |
|---|---|
| Timed collected urine | Albumin excretion 20 - 200 μg/min |
| Urine collection for 24 hr | Albumin excretion 30 - 300 mg/24hr |
| Spot urine sample (preferably first morning urine) | Albumin concentration 20 - 200 mg/l |
Etiology of Microalbuminuria: Micro-albuminuria is most frequently caused by kidney damage from diabetes. However, many other conditions can lead to kidney damage, high blood pressure, heart failure, cirrhosis or systemic lupus erythematosus (SLE). If early kidney damage is not treated, larger amounts of albumin and protein may leak into the urine. This condition is called macro-albuminuria or proteinuria. When the kidneys spill protein, it can mean serious kidney damage is present. This can lead to chronic kidney disease. The development of micro-albuminuria probably involves both metabolic and haemodynamic factors affecting renal microcirculation. On the other hand, sustained hypertension is known to cause transcapillary escape of proteins such as albumin by increasing the intra-glomerular pressure. On the other hand, metabolic disorders directly affect the glomerular basement membranes and their permeability, thereby altering glomerular function and ultimately causing glomerular sclerosis[57,58].
Haemodynamic aspects: Glomerular hydrostatic pressure is normally regulated by the relative vasoconstriction-vasodilation of the blood vessels leading to and from the glomerulus. Defects of this autoregulatory function may lead to increased glomerular hydrostatic pressure and increased urinary albumin excretion[57,65].
Metabolic aspects: Microalbuminuria could also be due to a loss of the anionic charge of the glomerular basement membrane. This has been observed in diabetic patients, in whom Advanced Glycosylation End products (AGE products) may bind to and neutralize the anionic proteins of the basement membrane, with concurrent increase in the transmembrane passage of albumin. In type 1 diabetes, urinary albumin excretion correlates with the deposits of AGE products. The risk of micro-albuminuria has been found to increase with the amount of glycosylated hemoglobin, HbA1c, in blood[66,67].
Metabolic syndrome: Microalbuminuria often occurs together with the metabolic syndrome consisting of hyperinsulinaemia and insulin resistance, increased triglyceride and decreased high-density lipoprotein (HDL) levels, hyperglycemia and hypertension.
Complications associated with microalbuminuria: Microalbuminuria is a reliable indicator of a risk of progressive renal and cardiovascular disorders. In 80 % of people with type 1 diabetes and microalbuminuria, urinary albumin excretion increases at a rate of 10 - 20 % per year, with development of clinical proteinuria (>300 mg albumin/day) in 10 - 15 years. After development of clinical grade proteinuria, most (> 80 %) patients go to develop decreased glomerular filtration rate (GFR) which lead to end-stage renal disease[59].
Some important consequences associated with micro-albuminuria are follows:
1. Increased risk of cardiovascular disease.
2. Increased risk of early mortality in patients with acute myocardial infarction.
3. Pregnancy complications and kidney dysfunction in hypertensive patients
Diagnostic criteria for microalbuminuria: Urinary albumin excretion has a high intra-individual variability, even when individuals refrain from physical exercise on the day before the collection of urine and when overnight collections are used to avoid acute variations from dietary protein. Therefore multiple measurements are usually required to make a diagnosis and repeated collections over time are required to establish the progression of albumin excretion over time. A result is considered to be diagnostic when two out of three urine specimens are positive within a 3-month period[64].
Sometimes it is more practical to test early morning urine samples and to measure the albumin/creatinine ration. Microalbuminuria is considered to be present when the ratio of albumin/Creatinine in urine reaches a level greater than 30 mg/g. This method may theoretically avoid falsely negative results due to polyuria and spurious reductions in urinary albumin concentration[13,64]. Generally, screening for microlabuminuria can be performed by three methods:
a. Measurement of albumin concentration in first-void morning urine or measurement of the albumin to Creatinine ratio in a random spot
b. 24-hour urine collection with Creatinine measurement, allowing simultaneous determination of Creatinine clearance
c. Timed (e.g. 4-hour to overnight) urine collection.
The first method is often found to be the easiest to carry out and generally provides accurate information. General criteria for abnormalities in albumin excretion are shown in table 4[64].
Table 4: Definitions of abnormalities in albumin excretion.
| Category | 24-h collection (mg/24 h) | Timed collection (μg/ min) | Spot collection(μg /mg creatinine) |
|---|---|---|---|
| Normal | < 30 | < 20 | < 30 |
| Microalbuminuria | 30 - 299 | 20 - 199 | 30 - 299 |
| Clinical albuminuria | > 300 | >200 | > 300 |
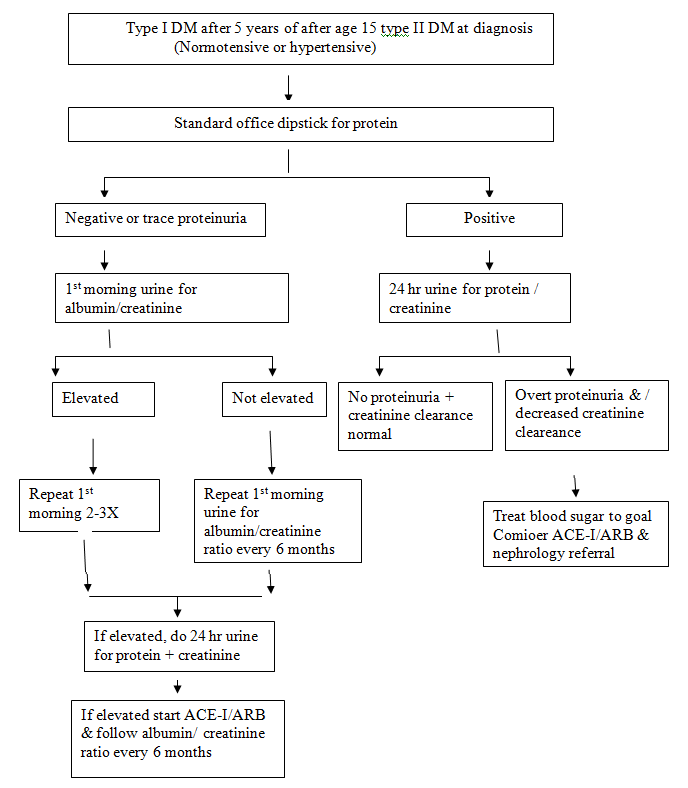
Figure 1: Microalbuminuria testing in diabetics.
Management: Glycemic control can prevent progression to microalbuminuria. Preventing the progression of each step of renal disease in patients with diabetes microalbuminuria, diabetic nephropathy, and ESRD or death can be achieved with blood pressure control[68] and the use of antiangiotensin therapies such as angiotensin-converting-enzyme (ACE) inhibitors and angiotensin II receptor blockers.
Primary prevention (preventing microalbuminuria): It can be achieved through good glycemic 26 and blood pressure control 30 and through the use of an ACE inhibitor in both type 1 and type 2 diabetes[69,70].
Secondary Prevention (preventing the progression from microalbuminuria to diabetic nephropathy): It can be achieved with an ACE inhibitor in both type 1 and type 2 diabetes[71-74] and with an angiotensin II receptor blocker 40 in type 2 diabetes.
Tertiary prevention (preventing the progression from diabetic nephropathy to ESRD): Independent of the blood pressure effect can be achieved with an ACE inhibitor in type 1diabetes and with an angiotensin II receptor blocker in type 2 diabetes[75,76]. It is unknown whether ACE inhibitors and angiotensin II receptor blockers are equally effective or whether they are more effective when combined. Once microalbuminuria is diagnosed in a patient with diabetes, it is time to stress to the patient the need to manage multiple risk factors for cardiovascular disease. The target blood pressure should be below 130/80 mm Hg[77] the target low-density lipoprotein cholesterol level should be below 2.5 mmol/L[78] and smoking cessation should be mandatory.
Conclusion
Diabetes is a common disorder and its frequency is dramatically rising around the world. The global increases in diabetes occur because of population ageing and growth, increasing trends towards obesity, unhealthy diets and sedentary lifestyles. Diabetes requires daily self care, if not then complications may develop which have a significant impact on quality of life and can reduce life expectancy. Worldwide, type 2 DM imposes heavy health burdens and its related complications. The main cause of the diabetes epidemic is the interaction between genetic and environmental risk factors. Microalbuminuria reflects vascular damage and appears to be a marker of early arterial disease and endothelial dysfunction. Intensive treatment with diabetes delays the onset of short-term, long-term and infectious complications. Therefore, it is important to screen microalbuminuria in urine using the urine albumin level in predicting development and progression of diabetic nephropathy. After the detection of microalbuminuria in a diabetic patient, the effective management of blood glucose concentration should be elevated, hypertension should be optimally treated, the patient should be encouraged to give up smoking and the intake of dietary protein should be restricted.
Conflict of interest:
Authors declared that there is no conflict of interest
References
- 1. Zimmet, P. Diabetes epidemiology as a trigger to diabetes research and care. (1999) Diabetologia 42(5): 499–518.
Pubmed || Crossref || Others - 2. Zimmet, P., Lefebvre, P. The global NIDDM epidemic. Treating the disease and ignoring the symptom. (1996) Diabetologia 39(11): 1247–1248.
Pubmed || Crossref || Others - 3. Amos, A., McCarty, D., Zimmet, P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. (1997) Daibet Med 14(5): S1-85.
Pubmed || Crossref || Others - 4. King, H., Aubert, R., Herman, W. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. (1998) Diabet Care 21(9):1414-1431.
Pubmed || Crossref || Others - 5. Murray, J.G. An approach to bacterial standards. (2002) J Appl bacteriol 32(2): 123-135.
Pubmed || Crossref || Others - 6. Alberti, K.G., Zimmet, P., Defronzo, R.A., et al. International textbook of diabetes mellitus. 2nd ed., Chichester. (1997)Wiley 1379.
Pubmed || Crossref || Others - 7. Mogensen, C.E., Christensen, C.K., Vittinghus, E. The stages in diabetic renal disease: with emphasis on the stage of incipient diabetic nephropathy. (1983) Diabetes 32(Suppl 2): 64-78.
Pubmed || Crossref || Others - 8. West, I.C. Radicals and oxidative stress in diabetes. (2000) Diabet Med 17(3): 171–180.
Pubmed || Crossref || Others - 9. Yadav, D.P., Prakash, S., Sharma, S., et al. Biochemical Analysis of Peroxynitrite Modified Human Serum Albumin (PN-HSA) in Rheumatoid Arthritis and Type I Diabetes. (2015) Int J pharmacy and pharmaceutical sci 4(2): 193-206.
Pubmed || Crossref || Others - 10. Michael, T., et al. Diabetics and cardiovascular disease. 2nd edition. (2005) Johnstone and aristidis veves-Human Press.
Pubmed || Crossref || Others - 11. William, A.P. Emerging antimicrobial resistance in the surgical compromised host. (1999) J chemother 11(6): 518-523.
Pubmed || Crossref || Others - 12. 2000 CORR report - dialysis and renal transplantation, Volume I. Ottawa: Canadian Institute for Health Information (2001).
Pubmed || Crossref || Others - 13. Prakash, S., Yadav, K., Singh, J.K., et al. Biochemical Perspectives of Microalbuminuria in Diabetes Mellitus as Early Risk Markers of Nephropathy. (2015) Asian journal of Biomedical Res 1(3): 1-4.
Pubmed || Crossref || Others - 14. Yadav, K., Prakash, S. Antimicrobial Resistance Pattern of Uropathogens Causing Urinary Tract Infection (UTI) Among Diabetics. (2016) Bio-medical Res Int 1: 07-15.
Pubmed || Crossref || Others - 15. Eastman, R.C., Keen, H. The impact of cardiovascular disease on people with diabetes: the potential for prevention. (1997) Lancet 350(Suppl 1): SI29-32.
Pubmed || Crossref || Others - 16. Warram, J.H., Gearin, G., Laffel, L., et al. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. (1996) J Am Soc Nephrol 7(6): 930-937.
Pubmed || Crossref || Others - 17. Nielsen, S., Schmitz, A., Rehling, M., et al. The clinical course of renal function in NIDDM patients with normo- and microalbuminuria. (1997) J Int Med 241(2): 133-141.
Pubmed || Crossref || Others - 18. Bennett, P.H., Haffner, S., Kasiske, B.L., et al. Screening and management of microalbuminuria in patients with diabetes mellitus. Recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. (1995) Am J Kidney Dis 25(1): 107-112.
Pubmed || Crossref || Others - 19. Delcourt, C., Vauzelle-Kervroedan, F., Cathelineau, G., et al. Low prevalence of long-term complications in non-insulin-dependent diabetes mellitus in France: a multicenter study. CODIAB-INSERM-ZENECA Pharma Study Group. (1998) J Diabet Complications 12(2): 88-95.
Pubmed || Crossref || Others - 20. Passa, P., Chatellier, G. The DIAB-HYCAR Study. (1996) Diabetologia 39(12): 1662-1667.
Pubmed || Crossref || Others - 21. Krolewski, A.S., Warram, J.H., Freire, M.B. Epidemiology of late diabetic complications. A basis for the development and evaluation of preventive programs. (1996) Endocrinol Metab Clin North Am 25(2): 217-242.
Pubmed || Crossref || Others - 22. Esmatjes, E., Castell, C., Gonzalez, T., et al. Epidemiology of renal involvement in type II diabetics (NIDDM) in Catalonia. The Catalan Diabetic Nephropathy Study Group. (1996) Diabetes Res Clin Pract 32(3): 157-163.
Pubmed || Crossref || Others - 23. Lievre, M., Marre, M., Chatellier, G., et al. The non-insulin-dependent diabetes, hypertension, microalbuminuria or proteinuria, cardiovascular events, and ramipril (DIABHYCAR) study: design, organization, and patient recruitment. DIABHYCAR Study Group. (2000) Control Clin Trials 21(4): 383-396.
Pubmed || Crossref || Others - 24. Pedrinelli, R., DellOmo, G., and Bello, V. Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. (2002) J Hum Hypertens 16(2): 79-89.
Pubmed || Crossref || Others - 25. WHO, Clinical Chemistry, WHO technical report series. (1985) 436.
Pubmed || Crossref || Others - 26. Eisenbarth, S. Prevention of Type I diabetes and recurrent b-cell destruction of transplanted islets. (1997) Endocr Rev18(2): 241-258.
Pubmed || Crossref || Others - 27. Paul, Z., Alberti, K.G., Shaw, J. Global and Societal implications of the diabetes epidemic. (2001) Nature 414(6865): 782-787
Pubmed || Crossref || Others - 28. De-Courten, M., Bennett, P.H., Tuomilehto, J., et al., in International Textbook of Diabetes Deckert, T., Feldt-Rasmussen, B., Djurup, R., Deckert, M. Glomerular size and charge selectivity in insulin-dependent diabetes mellitus. (1988) Kidney Int 33: 100-106.
Pubmed || Crossref || Others - 29. Mitanchez, D., Doiron, B., Chen, R., et al. “Glucose-stimulated genes and prospects of gene therapy for Type I diabetes.” (1997) Endocr Rev 18(4): 520-540.
Pubmed || Crossref || Others - 30. Bach, J.F. Insulin-dependent diabetes mellitus as an autoimmune disease. (1994) Endocr Rev 15(4): 516-542.
Pubmed || Crossref || Others - 31. Uomilehto, J., Tuomilehto-Wolf, E., Zimmet, P., et al., in International Textbook Vernier, R,L., Steffes, M.W., Sisson-Ross, S., Mauer, S.M. Heparan sulfate proteoglycan in the glomerular basement membrane in type 1 diabetes mellitus. (1992) Kidney Int 41: 1070-1080.
Pubmed || Crossref || Others - 32. Tominaga, M., et al. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not Tomlanovich, S., Deen, W.M., Jones, H.W III, Schwartz, H.C., Myers, B.D. Functional nature of glomerular injury in progressive diabetic glomerulopathy. (1987) Diabetes 36: 556-565.
Pubmed || Crossref || Others - 33. Astrup, A., Finer, N. Redefining type 2 diabetes: ‘diabesity’ or ‘obesity dependent diabetes mellitus’?. (2000) Obes Rev 1(2): 57–59.
Pubmed || Crossref || Others - 34. Yadav, B., Prakash, S., Sah, P., et al. Knowledge of Type-II Diabetes Mellitus and its complications among population of Siraha District, Nepal. (2016) Int J Innovative Applied Res 4(8): 19- 30.
Pubmed || Crossref || Others - 35. Tuomilehto, J., Lounamaa, R., Tuomilehto-Wolf, E., et al. Epidemiology of childhood diabetes mellitus in Finland-background of a nationwide study of type 1 diabetes (insulin dependent) diabetes mellitus. (1992) Diabetologia 35(1): 70–76.
Pubmed || Crossref || Others - 36. Atkinson, M., Maclaren, N. The pathogenesis of insulin-dependent diabetes mellitus. (1994) N Engl J Med 331(21): 1428–1436.
Pubmed || Crossref || Others - 37. Herold, K., Bloch, T., Vezys, V., et al. Diabetes induced with low doses of streptozotocin is mediated by V beta 8.2+ cells. (1995) Diabetes 44(3): 354–359.
Pubmed || Crossref || Others - 38. Harding, J. The nutritional basis of the fetal origins of adult disease. (2001) Int J Epidemiol 30(1): 15–23.
Pubmed || Crossref || Others - 39. Hattersley, A., Tooke, J. The fetal insulin hypothesis: an alternative explanation of the association of low birth weight with diabetes and vascular disease. (1999) Lancet 353(9166): 1789-1792.
Pubmed || Crossref || Others - 40. Joseph, K., Kramer, M. Review of the evidence on fetal and early childhood antecedents of adult Causes of insulin resistance. (1995) Nature 373: 384-385.
Pubmed || Crossref || Others - 41. Evephart, J.E., Pettit, D.J., Bennett, P.H., et al. Duration of obesity increases the incidence of NIDDM. (1992) Diabetes 41(2): 235-240.
Pubmed || Crossref || Others - 42. Taylor , V., Barr, V., Reitman , M. Does leptin contribute to diabetes caused by obesity? (1996) Science 274(5290): 1151-1152.
Pubmed || Crossref || Others - 43. Savage, M., Kilvert, A. ABCD guidelines for the management of hyperglycaemic emergencies in adults. (2006) Pract Diabetes Int 23(5): 227–231.
Pubmed || Crossref || Others - 44. Sampson, M.J., Brennan, C., Dhatariya, K., et al. A national survey of inpatient diabetes services in the UK. (2007) Diabet Med 24(6): 643–649.
Pubmed || Crossref || Others - 45. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. (1993) N Engl J Med 329: 977- 986.
Pubmed || Crossref || Others - 46. Herbal, G., Boyle, P. Hypoglycemia and hyperglycemia-pathophysiology and treatment. (2000) Endocrinol clin N Am 29: 725-745.
Pubmed || Crossref || Others - 47. Golden, S.H., Peart-Vigilance, C., Kao, W.H., et al. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes (1999) Diabetes care 22(9): 1408-1414.
Pubmed || Crossref || Others - 48. Ulaeto, D., Lacy, P.E., Kipnis, D.M., et al. A T-cell dormant state in the autoimmune process of non-obese diabetic mice treated with complete Freund's adjuvant. (1992) Proc Natl Acad Sci USA 89(9): 3927-3931.
Pubmed || Crossref || Others - 49. Fajans, S.S., Graeme, I.B., Polonsky, K.S. Molecular mechanisms and clinical pathophysiology of the maturity onset diabetes of the young. (2001) N Engl J Med 345(13): 971-980.
Pubmed || Crossref || Others - 50. Tattershall, R.B. Maturity-onset diabetes of the young (MODY) in Pickup and Williams. Textbook of Diabetes. (1991) 1:246.
Pubmed || Crossref || Others - 51. Stephan, C., Hauser-Darrel, S., Pardi-John, J. Potrucha-Mayo Clinics gastroenterology and hepatology broad review, 2nd Ed. (2005) Scientifics Press.
Pubmed || Crossref || Others - 52. Baldamus, C.A., Galaske, R., Eisenbach, G.M., et al. Glomerular protein filtration in normal and nephritic rats. A micropuncture study. (1975) Contrib Nephrol 1: 37-49.
Pubmed || Crossref || Others - 53. Reutens, A.T., Prentice, L., Atkins, R. The Epidemiology of Diabetic Kidney Disease, In: Ekoe J, editor. The Epidemiology of Diabetes Mellitus, 2nd Edition. (2008) Chichester: John Wiley & Sons Ltd. 499-518.
Pubmed || Crossref || Others - 54. Adler, A.I., Stevens, R.J., Manley, S.E., et al. Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). (2003) Kidney int 63(1): 225-232.
Pubmed || Crossref || Others - 55. Kimmelstiel, P., Wilson., C. Benign and malignant hypertension and nephrosclerosis. A clinical and pathological study. (1936) Am J pathol 12(1): 45-48.
Pubmed || Crossref || Others - 56. Myers, B.D., Nelson, R.G., Bennett, P.H., et al. Diabetic Renal Disease Study: Glomerular function at onset of nephropathy in NIDDM. (1991) J Am Soc Nephrol 2: 295.
Pubmed || Crossref || Others - 57. Deckert, T., Kofoed-Enevoldsen, A., Vidal, P., et al. Size- and charge selectivity of glomerular filtration in type 1 (insulin-dependent) diabetic patients with and without albuminuria. (1993) Diabetologia 36(30): 244-251.
Pubmed || Crossref || Others - 58. Friedman, S., Jones, H.W., Golbetz, H.V., et al. Mechanisms of proteinuria in diabetic nephropathy. II. A study of the size-selective glomerular filtration barrier. (1983) Diabetes 32 (Suppl. 2): 40-46.
Pubmed || Crossref || Others - 59. Mogensen, C.E. Microalbuminuria, blood presure and diabetic renal disease: origin and development of ideas. (1999) Diabetologia 42(3): 263-285.