New Lipopolysaccharide Binding Proteins from the Jellyfises Aurelia Aurita and Rhopilema Asamushi of Sea of Japan
Affiliation
- 1G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of Russian Academy of Sciences, Vladivostok, Russia
- 2Far Eastern Federal University, School of Biomedicine, Vladivostok, Russia
Corresponding Author
G.A. Naberezhnykh, G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far East Branch of the Russian Academy of Sciences, Pr. 100-letiya Vladivostoka, 159, Vladivostok, Russia, 690022, Tel: +7-4232-311406/ Fax: +7-4232-314050; E-mail: naber1953@mail.ru
Citation
Naberezhnykh, G.A., et al. New Lipopolysaccharide Binding Proteins from the Jellyfishes Aurelia Aurita and Rhopilema Asamushi of Sea of Japan. (2017) J Marine Biol Aquacult 3(2): 1- 5.
Copy rights
© 2017 Naberezhnykh, G.A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
marine invertebrates, innate immunity, scyphozoan jellies, lipopolysaccharides, lipopolysaccharide binding proteins
Abstract
The commercial species of jellyfishes of Sea of Japan were studied as the sources of proteins that are capable lipopolysaccharides (LPSs) of Gram-negative bacteria. LPS-binding proteins have been found in lysates of jellyfishes mesogloea by means of immunofluorescence assay using LPS labeled with fluorescein (F-LPS). It was shown that lysate from rhopilema demonstrate the major LPS-binding activity in comparison with extracts from aurelia. For isolation and purifying of LPS-binding proteins from lysate of jellyfishes, the cation-exchange chromatography was used. It was shown, that fractions of jellyfishes lysate after cation-exchange chromatography contained several LBP with high-molecular weights. By ligand enzyme solid phase assay it was shown that isolated LBP of jellyfishes was bound to LPS directly by concentration-dependent manner in saturation process.
Introduction
Invertebrates, in contrast of vertebrate animals, have no acquired immunity, therefore for protection against intussusceptions of foreign agents they use innate immunity system[1]. The part of ancient protective system forming the basis of nonspecific immunity of invertebrates, has served as the precursor of the complicated immunity of vertebrate animals[2]. The antimicrobial function of the innate immunity is realized by using specific “recognition” proteins known as pattern recognition proteins (PRPs) that bind to specific molecules on the surface of pathogens also been called as pathogen-associated molecular patterns (PAMPs) to activate the entire immune system[1]. In Gram-negative bacteria, one of the PAMP compounds is the lipopolysaccharide (LPS). A LPS molecule has an amphiphilic nature and consists of three parts: lipid A, core oligosaccharide, and the O-polysaccharide chain. Proteins that “recognize” LPSs are called LPS-binding proteins (LBP) which are capable interact with both lipid and the carbohydrate moieties of the LPS molecule[3,4].
Currently known LPS-binding proteins were found in species that inhabit in the Southeast Asia tropical seas[5-7]. Such proteins have not been previously sought in invertebrates of the northern seas. Now LBP of rather small number of invertebrates species are studied, mainly on the crustaceans and mollusca of tropical seas. Basically, they are proteins with molecular weights of 40 to 175 kDa. It is determined that LBP are synthesized in various tissues of invertebrates, but mostly found in blood cells[8]. In experiences of pathogenic bacteria infection on crustaceans with have shown that LPS-binding proteins are inducible with expression increases greatly within 8 - 12 hours after infects animals with Gram-negative bacteria or LPS administration[9]. In invertebrates, binding of these proteins with LPS promote protective mechanisms such as haemolymph coagulation[10] and activation of phenoloxydase system[11].
Study of the proteins that play an important role in the protection against bacterial infections will contribute to a better understanding of the innate immune system function in invertebrates and thus would help to develop a new generation of antimicrobial preparations. The need for such preparations in invertebrates is currently important because of the development of mariculture. Moreover, LPS-binding proteins are of interest as potential agents for the treatment of endotoxemia and Gram-negative sepsis in humans[12].
From jellyfish Aurelia aurita, the cationic peptide possessing antimicrobial and membrane-bound properties potentially capable to interlink with LPS was isolated and described[13]. Recently we discovered variety of invertebrates of Sea of Okhotsk can be considered as new sources of high-molecular LBP[14].
The purpose of the present work is to study the presence of LPS-binding proteins in the jellyfishes Rhopilema asamushi (rhopilema) and Aurelia aurita (aurelia) in the Sea of Japan. Undertaken study will allow identifying of perspective jellyfishes species for isolation of these proteins for further study.
Materials and Methods
Raw material
Jellyfishes were collected in Ussuriisk bay of Sea of Japan in the autumn season (from September to October) and stored in refrigerator at -18°C. Lysates from R. asamushi and A. aurita were obtained by 3-fold freeze-thawing process. Insoluble fraction was removed by centrifugation and supernatant fluid concentrated by ultrafiltration on the membrane with exception limit of 3 kDa (Millipore, USA) followed by dialyzed against PBS. The concentration of the total protein in the lysates of jellyfishes was determined by the Bradford method[15].
Preparation of fluorescein and biotin labeled LPS[16]
A total of 30 mg of LPS E. ?oli 055 B5 (Flu?a, Germany) were dissolved in 1 ml of sterile deionize water. For disaggregation of LPS, 2 μL of triethylamine was added and solution was treated on ultrasonic bath for 10 min. For introduction of amino groups in polysaccharide part of LPS, 20 mg of cyanogen bromide in 50 μL of acetonitrile was added. The mixture was incubated for 10 minutes and ethylenediamine (30 mg in 0.02 M NaHCO3) was added. After 60 minutes of incubation, solution was dialyzed for 48 hours against water and lyophilized. As a result, 25 mg of Amino-LPS was obtained.
For synthesis of lipopolysaccharide labeled with fluorescein (F-LPS), 20 mg of Amino-LPS was dissolved in 3 ml of 0.02 M NaHCO3 (?? 9.5), solution of 4 mg fluorescein isothiocyanate (Sigma, USA) in 2.5 mL of dimethylsulphoxide was added and mixture was incubated for overnight at 37°?. The reaction mixture was dialyzed for 48 hours against water and lyophilized.
For introduction of biotin, 1 mg of N-hydroxysuccinimide ester of biotinamidohexanic acid (Sigma, USA) dissolved in 0.1 mL of dimethyl sulfoxide was added to 6 mg of amino-LPS in 1 mL of 0.1 M NaHCO3 (pH 8.3); mixture was incubated for 16 hours at 37°C. The resulting LPS labeled with biotin (B-LPS) was dialyzed for 48 hours against water and lyophilized.
Detection of LPS-Binding Proteins by DOT Analysis
The presence of LPS-binding proteins in the lysates of jellyfishes was determined by express DOT analysis using F-LPS. Sample of 5 μL (concentration of the total protein 2 mg.mL-1) lysates were applied to nitrocellulose membrane (0.2 μm, Sartorius, Germany), air dried and fixed with 0.25 % solution of glutaraldehyde in water for 30 minutes. To block the nonspecific binding sites, membrane was treated with phosphate-saline buffer (PBS: 0.01 M NaH2PO4 + 0.15 M NaCl; pH 7.5), containing 0.25 % Tween-20 and 10 mg.mL-1 BSA (PBS-T). Incubation of nitrocellulose with F-LPS (20 μg. ml-1 in PBS-T) was performed for 2 hours at 37°C. For visualization of spots were count under NU-6KL fluorescent lamp (Germany). Polymyxin B (Pm-B) was used as a positive control.
Detection of LPS-binding proteins by agglutination LPS - latex
Amino-LPS (5 mg.mL-1 in deionize water) was bounded with 200 μL activated polystyrene latex preliminarily flushed with 0.2 M NaHCO3 ?? 9.5. Latex was washed off with PBS-? from the not bounded LPS and suspended in 10 ml of PBS-T. Lysates of jellyfishes were 2-fold titrated in round bottomed dishes and 50 μL of LPS-latex was added. Results were registered by forming of “umbrella” - positive or “points” – negative results. The maximum dilution of extract with positive result was considered as extract titer.
Detection of LBP by the ligand-enzyme solid-phase assay
A total of 100 μL pooled fractions sample after cation- exchange chromatography (concentration of the total protein 20 μg.mL-1) or polymyxin B in same concentration was placed into the wells of polystyrene plate PolySorp (Nunc, USA) and incubated for 16 hours at 37°C. Non-specific binding sites were blocked with PBS-T, containing 1% BSA. The wells filled with 100 μL of PBS-T were used as blank, and those with 100 μL PBS-T containing 100 μg.mL-1 BSA were used as a negative control. The plates were washed with PBS-T and wells were filled with 100 μL of B-LPS solution in different concentrations (0.08 – 2.0 mg.mL-1) and incubated for 4 hours at 37°C. After incubation, the plates were washed with PBS-T, the conjugate of horse radish peroxidase and streptavidin in dilution of 1:1000 was added into the wells followed by incubated for 1 hour at 37°C. The amount of the bound B-LPS was determined by measuring the optical density of solution after addition of substrate to enzyme. O-phenylenediamine was used as a chromogen in the substrate mixture. The absorbance of reaction mixture was determined with μQuant Bio-TEK Instruments spectrophotometer (INC, USA) at a wavelength of 492 nm.
FPLC ion-exchange chromatography of jellyfishes extracts
Chromatography was carried out on FPLC-chromatograph (Amersham Pharmacia Biotech, Sweden) on cation exchange column Sourse 15S. Sample of 5 mL of jellyfishes lysates were applied, not bounded protein was washed off with 0.02 M phosphate buffer at ?? of 6.8, cationic proteins were eluted with linear gradient of 2 M NaCl in phosphate buffer (?? 6.8), collecting fractions by 1 mL. Absorbance of fractions at 280 nm was determined and checked on LPS-binding activity by means of F-LPS. The active fractions were combined and concentrated by ultrafiltration on the membrane with exception limit of 3 kDa (Millipore, USA) and dialyzed against PBS.
Polyacrylamide Gel Electrophoresis of Proteins Followed by Electrotransfer to Nitrocellulose Membrane
LBP of jellyfishes after ion-exchange chromatography were separated by SDS-PAAG-electrophoresis on 12 % PAAG according to the method of Laemmli[17]. Samples were prepared according to two methods: without warming-up at 100°? and prior heating of the sample (5 min at 100°?) in a buffer containing 2 % sodium dodecyl sulfate (SDS) and β-mercaptoethanol. The set of colored proteins (Fermentas, Lithuania) with molecular weights of 11, 17, 24, 33, 40, 55, 72, 100 and 130 kDa were used as markers. Proteins separated in gel were stained with solution of Coomassie R-250 in 10% acetic acid and 30% methanol.
For the Western blotting, proteins after electrophoresis were transferred on nitrocellulose (0.2 μm, Sartorius, Germany) followed standard procedure[18]. To block the nonspecific binding sites, membrane was treated with PBS containing 0.25% Tween-20 and 1% BSA. Detection of LPS-binding proteins on blots was performed using the F-LPS as described above the DOT analysis. Fluorescence of F-LPS-protein of complexes was registered by Versa Doc imaging system (Bio-Rad, USA).
Results and Discussion
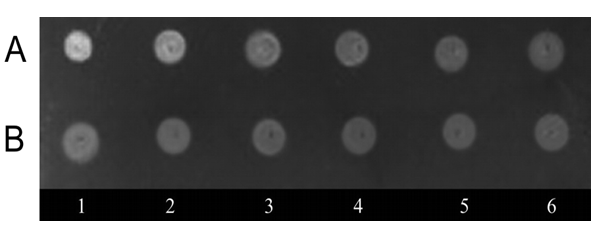
Lysates from R. asamushi and A. aurita were obtained by 3-fold freeze-thawing. By means of the DOT analysis using F-LPS, LBP have been found in extracts of both species of jellyfishes (Figure 1).
Figure 1: Quantitative DOT analysis of binding of F-LPS, (A) with lysates of R. asamushi, (B) with lysates of A. aurita with 1 – 6 serial twofold dilutions of the samples. Concentration of the total protein lysates 2 mgmL-1
It should be noted that extracts from rhopilema, in comparison with extracts from aurelia, showed the major LPS-binding activity that has been determined by intensity of fluorescence of complexes F-LPS-protein in the DOT analysis.
Besides, LPS-binding activity of jellyfishes extracts was determined by means of agglutination of the latex loaded with LPS. It was shown that titers of rhopilema extracts were 1:8 which was more than of aurelia - 1:4.
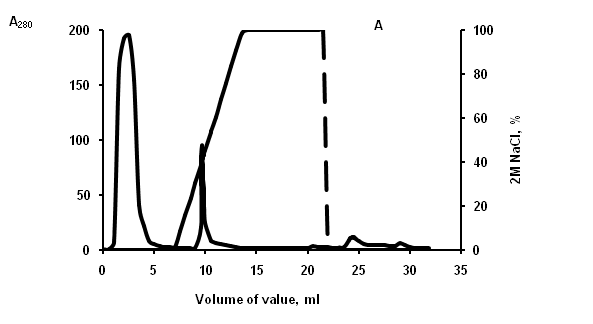
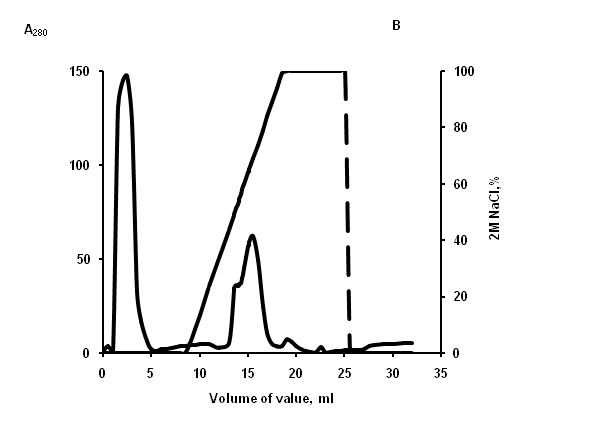
Figure 2: Elution profile from cation-exchange column. (A) lysate of aurelia, (B) lysate of rhopilema. Unbound material was washed extensively 20 mM phosphate buffer, and then adsorbed material was eluted with gradient 2? NaCl in the same buffer (dash line). Fractions were monitored at 280 nm (solid line) and tested for lipopolysaccharide-binding activity.
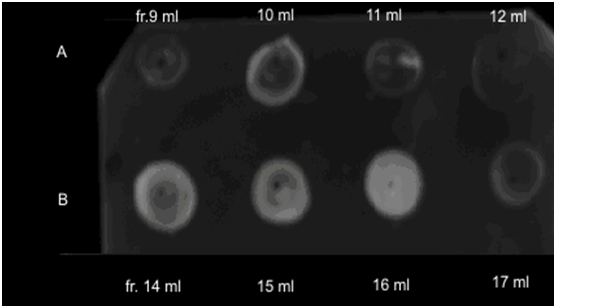
For purifying of LBP from jellyfishes extracts, the cation-exchange chromatography was used (Figure 2). DOT analysis data, LPS-binding activity has been found in fractions eluted in the field of concentration gradient of 0.85 and 1,25 ? NaCl for aurelia and rhopilema, respectively (Figure 3), that testifies to different positive charge on proteins of this fraction.
Figure 3: LPS-binding activity of fractions after cation-exchange chromatography of aurelia (A) and rhopilema (B) lysates.
The active fractions were combined and concentrated by ultrafiltration on the membrane with exception limit of 3 kDa. Activity in these filtrates was not found that indicated lack of LPS-binding activity at low molecular mass peptides.
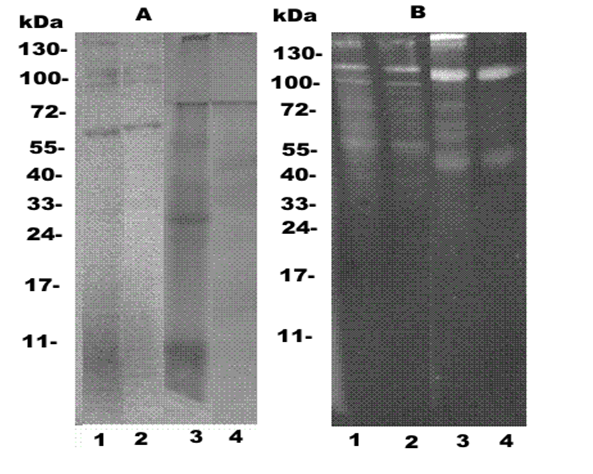
Proteins pooled fractions after cation-exchange chromatography of lysates in these species were separated by SDSPAAG electrophoresis and LBP were detected by Western blotting after treatment by F-LPS. The explored fractions were differ against each other by both amount, and molecular masses of proteins (Figure 4A). From the electrophoregrams of aurelia, several bands slightly colored by Coomassie in gel top were observed (Figure 4?, track 1). Western blotting of this sample detected two intensive (100 and 120 kDa) and two weak bands (55 and 130 kDa) of the proteins associated with a fluorescently labeled LPS (Figure 4B, tracks 1,2). Heating of the sample at 100°? in 2 % SDS, LPS-binding proteins were manifested in the not form of new polypeptides (Figure 4B, tracks 1,2).
Figure 4: Electrophoregram of jellyfishes proteins after cation-exchange chromatography. (A) - electrophoresis in PAAG; (B) - Western blot analysis of samples identified with fluorescein-labeled LPS. 1,3 - samples were prepared without warming-up at 100°?; 2, 4 - prior heating of the sample at 100°? in a buffer containing 2 % sodium dodecyl sulfate (SDS) and β-mercaptoethanol 1, 2,- A. aurita. 3, 4- R. asamushi. Left - molecular weights of proteins marker.
From the electrophoregram and Western blotting of rhopilema proteins we observed a different pattern of polypeptide distribution by size and activity. At the nondenaturing conditions of Western blotting, predominant polypeptides with molecular weights of 130,120 and 110 kDa and a minor band at 55 kDa demonstrated LPS-binding activity (Figure 4B, track 3) were observed. After heating to 100°?, LPS-binding proteins appeared as the predominant polypeptide as dominant band at 110 kDa and a minor band at 45 kDa (Figure 1?, track 4). Changes of the profiles of LPS binding rhopilema proteins in Western blot analysis after boiling in 2% SDS lysates can be a result of either dissociation or denaturation of proteins. The small difference in the apparent molecular weights of LBP before and after boiling supports their possible denaturation.
According to the literature data, marine invertebrates contain different LPS-binding proteins of high and low molecular weights, as oligomers and monomers. Thus, a protein with a molecular weight of 420 kDa that was capable of binding LPS was detected in the haemolymph of the shrimp Penaeus japonicus; during SDS-PAAG electrophoresis under denaturing conditions the protein dissociated to a monomer with a molecular weight of 32 kDa[19]. However, Luo et al. isolated LPS-binding protein with a low molecular weight of 18 kDa from Penaeus monodon and found that the weight did not change under denaturating electrophoresis conditions[20].
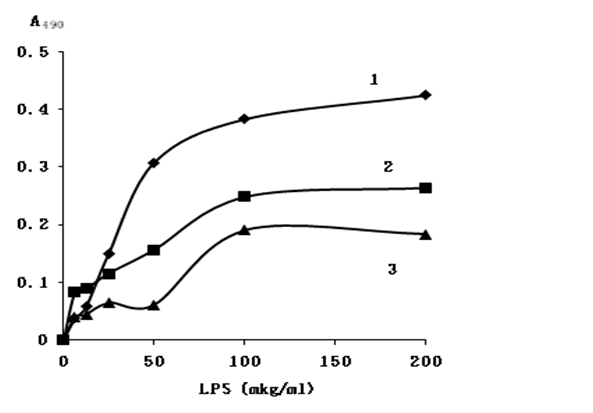
We used a ligand enzyme solid phase assay to study the interaction of LPS with proteins after cation-exchange chromatography. LBP of jellyfishes after chromatography with the same total protein concentrations were adsorbed on the surface of a polystyrol plate and titrated with biotin labeled LPS. A streptavidin– horseradish peroxidase conjugate was used to determine the resulting complexes. As it appears from figure 5, binding of proteins with LPS has a specific character, as saturation of binding sites on the proteins can be reached by the ligand. At the saturation point, the amount of LPS that was bound by the proteins was significantly smaller than that of polymyxin B (Figure 5, line 1). The difference in the binding activity of the proteins and polymyxin B might be due to a high constant of binding of polymyxin with LPS. This was in agreement with Vaara (1992) highlighted that PmB binding point can reaches to 2.5 x 107 M-1[21].
Figure 5: Binding of biotin-labeled LPS with immobilized on polystyrene plates: (1) polymyxin B, (1), proteins of rhopilema (2) and aurelia (3). Concentration of the total protein lysates and polymyxin B 20 μg.mL-1.
Conclusion
The LPS-binding proteins are present in lysates of two jellyfishes tissues the rhopilema in comparison with aurelia showed the major LPS-binding activity that has been determined by intensity of fluorescence of complexes F-LPS-protein in the DOT analysis. High-molecular weights LBP specifically binding with LPS was isolated by cation-exchange chromatography. These species of invertebrates are widely available and are of interest as a new source of LPS-binding proteins which are potential antimicrobial agents. In addition, we developed a highly sensitive DOT analysis for LPS-binding proteins determination in the extracts of invertebrates using fluorescently labeled LPS. This is a fairly quick method that does not require any sophisticated equipment and applicable in field conditions.
Acknowledge:
The work completed at partial financial support of Russian Science Foundation (RSF) and Far Eastern federal university (FEFU) grant «Time-lapse technology and rational use of marine biological resources» ? 14-50-00034 at the Direction ? 3 «Development of innovative pharmaceutical drugs and functional foodstuff».
References
- 1. Iwanaga, S., Lee, B.L. Recent advances in the innate immunity of invertebrate animals. (2005) J Biochem Mol Biology 38(2): 128-150.
Pubmed || Crossref || Others - 2. Beck, G., Habicht, G.S. Immunity and the Invertebrates. (1996) Sci Am 275(5): 60-63, 66.
Pubmed || Crossref || Others - 3. Chaby, R. Lipopolysaccharide-binding molecules: transporters, blockers and sensors. (2004) Cell Mol Life Sci 61(14): 1697-1713.
Pubmed || Crossref || Others - 4. Koshiba, T., Hashii, T., Kawabata, S. Structural perspective on the interaction between LPS and factor C, a receptor involved in recognition of Gram-negative bacteria. (2007) J Biol Chem 282(6): 3962-3967.
Pubmed || Crossref || Others - 5. Kawabata, S., Muta, T. Sadaaki Iwanaga: discovery of the lipopolysaccharide - and β-1, 3-D-glucan-mediated proteolytic cascade and unique proteins in invertebrate immunity. (2010) J Biochem 147(5): 611-618.
Pubmed || Crossref || Others - 6. Cheng, W., Liu, C.H., Tsai, C.H. Molecular cloning and characterization of a pattern recognition molecule, lipopolysaccharide and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. (2005) Fish Shellfish Immunol 18(4): 297-310.
Pubmed || Crossref || Others - 7. Vargas-Albores, F., Guzman, M.A., Ocha, J.L. A lipopolysaccharide-binding agglutinin isolated from brown shrimp (Penaeus californiensis Holmes) hemolymph. (1993) Comp Biochem Physiol B Biochem Mol Biol104(2): 407-413.
Pubmed || Crossref || Others - 8. Zhao, D., Chen, L., Qin, C. Molecular cloning and characterization of the lipopolysaccharide and β-1, 3-glucan binding protein in Chinese mitten crab (Eriochersinensis). (2009) Comp Biochem Physiol B Biochem Mol Biol 154(1): 17-24.
Pubmed || Crossref || Others - 9. Zhang, D., Ma, J., Jiang, J., Qiu, L. et al. Molecular characterization and expression analysis of lipopolysaccharide and β-1,3-glucan-binding protein (LGBP) from pearl oyster Pinctada fucata. (2010) Mol Biol Rep 37(7): 3335–3343.
Pubmed || Crossref || Others - 10. Yeh, M.S., Chang, C.C., Cheng, W. Molecular cloning and characterisation of lipopolysacccharide and β-1,3-glucan binding protein from the giant freshwater prawn Macrobrachium rosenbergii and its transcription in relation to foreign material injection and the moult stage. (2009) Fish Shellfish Immunol 27(6): 701-706.
Pubmed || Crossref || Others - 11. Lee, S.Y., Wang, R., Soderhall, K. A lipopolysaccharide - and β-1,3-glucan binding protein from hemocytes of the freshwater crayfish Pacifas tacusleniusculus. Purification, characterisation, and cDNA cloning. (2000) J Biol Chem 275(2):1337-1343.
Pubmed || Crossref || Others - 12. Mariottini, G.L. Grice, I.D. Antimicrobials from Cnidarians. A New Perspective for Anti-Infective Therapy? (2016) Mar Drugs 14(48): 2-19.
Pubmed || Crossref || Others - 13. Ovchinnikova, T.V., Balandin, S.V., Aleshina, G.M., et al. Aurelin, a novel antimicrobial peptide from jelly?sh Aurelia aurita with structural features of defensins and channel-blocking toxins. (2006) Biochem Bioph Res Com 348(2): 514-523.
Pubmed || Crossref || Others - 14. Bakholdina, S.I., Naberezhnykh, G.A., Gorbach, V.I., et al. Marine Invertebrates of the Sea of Okhotsk as a New Source of Lypopolysaccharide Binding Proteins. (2014) Russian J Mar Biol 40(1): 59-65.
Pubmed || Crossref || Others - 15. Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. (1976) Anal Biochem 7(2): 248–254.
Pubmed || Crossref || Others - 16. Battaglini, F., Pallarola, D. Two Efficient Methods for the Conjugation of Smooth-Form Lipopolysaccharides with Probes Bearing Hydrazine or Amino Groups. I. LPS Activation with Cyanogen Bromide (2011) Methods Mol Biol 739: 147-160.
Pubmed || Crossref || Others - 17. Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T 4. (1970) Nature 227(5259): 680-685.
Pubmed || Crossref || Others - 18. Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. (1979) Proc Natl Acad Sci USA 76(9): 4350-4354.
Pubmed || Crossref || Others - 19. Yeh, M.S., Chang, C.C., Cheng, W. Molecular cloning and characterization of lipopolysaccharide and β-1,3-glucan binding protein from the giant freshwater prawn Macrobrachium rosenbergii and its transcription in relation to foreign material injection and the moltstage. (2009) Fish Shellfish Immunol 27(6): 701–706.
Pubmed || Crossref || Others - 20. Luo, T., Yang, H., Li, F., et al. Purification, characterization and cDNA cloning of a novel lipopolysaccharide-binding lectin from the shrimp Penaeus monodon. (2006) Dev Comp Immunol 30(7): 607–617.
Pubmed || Crossref || Others - 21. Vaara, M. Agents that increase the permeability of the outer membrane. (1992) Microbiol Rev 56(3): 395-411.
Pubmed || Crossref || Others