Next-generation tumor profiling identified high total mutation burden (TMB) in Lymphoepithelioma-like carcinoma of the urinary bladder (LELCB): A potential rationale for Immunotherapy
Daniel Brunnhoelzl , Sheila Bhavsar , Richard Trepeta
Affiliation
1 Creighton University School of Medicine at St. Joseph’s Hospital and Medical Center, Phoenix, AZ
2 Department of Pathology, St. Joseph’s Hospital and Medical Center, Phoenix, AZ
3 Genitourinary Oncology Section, University of Arizona Cancer Center at Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, AZ
Corresponding Author
Jianli Dong, Molecular Diagnostics Division, Department of Pathology, University of Texas Medical Branch, 301 University Boulevard, Galveston, Texas 77555-0743, USA, Tel: (409) 772-4866, E-mail: jidong@utmb.edu
Citation
Wang, J., et al. Next-generation tumor profiling identified high total mutation burden (TMB) in lymphoepithelioma- like carcinoma of the urinary bladder (LELCB): A potential rationale for Immunotherapy. (2017) Clin Trials Pathol Case Stud 2(1): 66- 69.
Copy rights
© 2017 Wang, J. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Urinary bladder cancer (BC); Lymphoepithelioma-like carcinoma of the urinary bladder (LELCB); Next generation sequencing [NGS]; Tumor-infiltrating lymphocytes; Total mutation burden (TMB); Prognosis; Immune checkpoint inhibitors; Immunotherapy
Abstract
Urinary Bladder Cancer (BC) is a heterogeneous disease with diverse morphologic and clinical manifestations. Lymphoepithelioma-Like Carcinoma of the Urinary Bladder (LELCB) is rare form of BC with no standard therapies exist. Thus, novel therapies based on underlying tumor biology are needed. Molecular profiling can be used to detect mutational load and molecular subtypes of tumors, which may help in predicting a patient’s sensitivity to immunotherapy approaches, such as PD-1/PD-L1 inhibition. Here we present a case of Lymphoepithelioma-Like Carcinoma of the Urinary Bladder (LELCB) in a 67-year-old male who underwent Transurethral Resection of Bladder Tumor (TURBT), pathology revealed muscle-invasive urothelial carcinoma, lymph epithelial type. Next-Generation gene sequencing identified multiple gene alterations, and high Total Mutation Burden (TMB) in tumor specimens, suggesting targeting the PD-1/PD-L1 axis may be a therapeutic option. A further characterization and description of the outcomes of these rare tumors is warranted to help guide physicians and counsel patients.
Introduction
Lymphoepithelioma is an undifferentiated carcinoma of the nasopharynx, while Lymphoepithelioma-like carcinoma (LELC) is a tumor that resembles lymphoepithelioma in terms of pathological features. Pathological features include poorly differentiated epithelial cells with dense lymphoid cell infiltration. Since first described in the nasopharynx, LELC has been reported in multiple organ systems, such as head and neck, lung, cervix, stomach, skin, ovaries and breast[1-6]. Lymphoepithelio-ma-like carcinoma of the urinary bladder (LELCB) is an exceedingly rare variant of infiltrating urothelial carcinoma that account approximately 0.3 - 1.3% of all bladder malignancies[7-9]. Given its rarity, and owing to the scarcity of reported cases, the optimal treatment is yet to be defined.
Genetic profiling of tumors provides new avenues for treatment of the disease; next-generation molecular profiling may have a role in such rare neoplasms, by searching for sites of possible susceptibility to standard adjuvant therapies. Large comprehensive analyses such as that of The Cancer Genome Atlas have provided important clues into carcinogenesis and revealed additional potentially druggable targets for metastatic bladder cancer[10]. Von Hoff and associates[11] performed molecular profiling and treatment based on molecular profiling on 66 patients with multiple tumor subtypes (breast, colorectal, ovarian, and multiple rare malignancies). The authors demonstrated the ability to measure molecular targets in patients’ tumors, as well as to find potential therapeutic targets, and they observed a longer progression-free survival among patients treated on the basis of their molecular profiling when compared to their prior therapy. While the next-generation sequencing technology is becoming more accessible and widespread, molecular targets have not thoroughly proven to be reliable indicators of in vivo response to therapy. Despite this caveat, with rare tumors, for which large clinical trials are not feasible, molecular tumor profiling may represent a clinician’s best attempt at an informed opinion about therapies.
We present a new case of this rare entity with molecular characterization of tumor sample using next-generation molecular techniques.
Case Presentation
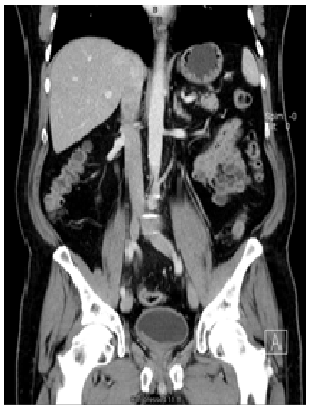
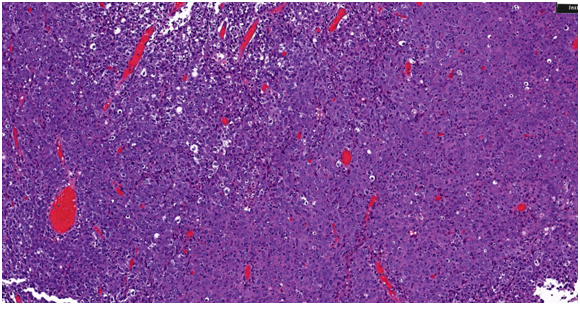
A 67-year-old male presented to his urologist complaining of gross hematuria beginning in November 2016. Digital rectal exam was performed and revealed no abnormalities, prostate specific antigen level was 0.4. Urine culture was found to be negative. Urine cytology and FISH were suspicious for malignancy. Computed tomography of the abdomen at an outside facility revealed 6.1 × 1.8 cm irregular mass within the urinary bladder without ureteral obstruction (Figure 1). There was no evidence of metastatic disease. Patient subsequently underwent transurethral resection of bladder tumor (TURBT) on 3/14/2017 and pathology revealed muscle-invasive urothelial carcinoma, lymph epithelial type (Figure 2).
Figure 1: A coronal computed tomography scan with contrast demonstrating a homogenous mass at the bladder neck. No metastases or lymphadenopathy were present.
Figure 2: Histopathological findings of the tumor underwent transurethral resection of bladder tumor (TURBT) resulting in diagnosis of lymphoepithelioma-like carcinoma of the urinary bladder (LELCB). Haematoxylin and Eosin (HE) staining showed that the tumor consisted of cells with irregular nucleus borders and reactive lymphoplasmacytic cells in both the tumor and stromal tissue.
Two weeks later a cystoscopy was performed, which showed inflammation and healing at the site of TURBT. After careful consideration, the patient decided to pursue an ongoing clinical trial at our institution: A Randomized Phase II Study of CO-eXpression ExtrapolatioN (COXEN) - Directed Neoadjuvant Chemotherapy for Localized, Muscle-Invasive Bladder Cancer (ClinicalTrials.gov Registry Number: NCT02177695). He was randomized to the Gemcitabine & Cisplatin arm, which includes Gemcitabine, 1000 mg/m², IV, Days 1 & 8, q21 days × 4 cycles; Cisplatin, 70 mg/m², IV, Day 1, q21 days × 4 cycles. He remains free of disease at last follow-up cystoscopy.
Next-generation Tumor Profiling
The tumor tissue was further evaluated with next-generation gene sequencing to evaluate for specific mutations. With hybridization capture of 3,769 exons from 315 cancer-related genes and introns of 28 genes commonly rearranged in cancer; ≥ 50 ng of DNA, sequenced to high (average 756X), uniform coverage. The mutational analysis revealed ten genes with known alterations (Table 1). Additionally, one or more Variants of Unknown Significance (VUS) in 39 genes were detected in this patient’s tumor, including: ATM, PMS2, MLH1, and MSH6. The Tumor Mutation Burden (TMB) was 50 Muts/Mb.
Table 1: Next-generation sequencing of tumor tissue from revealed 10 gene alterations.
| Gene | alterations |
|---|---|
| ERBB3 | G284R |
| ARID1A | Q1334_R1335insQ |
| AXIN1 | G738R |
| CHD2 | S23* |
| DNMT3A | E240fs*70 |
| MCL1 | amplification |
| PMS2 | E5* |
| TERT | promoter -124C >T |
| TP53 | Q331* |
| Tumor Mutation Burden | 50 Muts/Mb |
Discussion
Lymphoepithelial-like carcinomas are uncommon cancers. They are usually found in pharyngeal and foregut derivatives like salivary glands, thymus, stomach and liver. However, recent cases of LELC detected in other anatomical locations are also reported, such as bladder[1], lacrimal glands[2], ovaries[3], cervix[4], skin[5], breast[6] etc. Since the first reported case of LELC in the lower respiratory tract by Bégin[12], there have been approximately 140 reported cases of LELCB worldwide in the literature[13]. The mean age of presentation is 70 years[13]. Lymphoepithelial carcinoma of the nasopharynx is associated with infection with the Epstein Barr Virus (EBV); however, no infectious risk factor is known for LELCB. The exact pathogenesis of LELC remains unknown, but an abnormality in p53 regulation and stem cell origin for the carcinoma have been suggested[8-14].
The disease biology and clinical behavior of LELCB have not been well-established. Due to the inflammatory cell infiltrate observed upon histologic examination, LELC may appear similar to a lymphoma or chronic inflammatory reaction; however, cytokeratin stains reveal the presence of carcinoma cells. The most common presenting symptom is gross hematuria accompanied by urgency, and most cases are in T2-T3 stage at the time of diagnosis[15]. Amin et al. subdivided LELC by determining the percentage of LELC morphology within the tumor, characterizing pure (100%), predominant (> 50%) and focal (< 50%) disease[16]. A pooled analysis of 140 cases of LELCB found that the best treatment modality (associated with the highest disease-free survival) is multi-modal treatment including radical cystectomy[13]. In the case of muscle invasive disease, most authors recommended radical cystectomy[17]. However, benefits of neoadjuvant chemotherapy have been reported LELCB. For instance, Serrano et al conducted a pooled analysis of 56 patients and concluded that, whilst focal disease is more aggressive and requires a radical cystectomy, pure or predominant tumors are amenable to bladder-preserving treatment[18]. In the Serrano study, patients with pure/predominant LELCB who received systemic chemotherapy following TUR demonstrated a 100% disease-free survival, compared with 53% disease-free survival in those who did not, at a median follow-up of 34 and 25 months respectively[18,19]. DInney et al used platinum-based chemotherapies for three patients with muscle-invasive LELCB, with all patients remaining free of recurrence after six years of follow-up[19]. Similar findings were reported by Lopez and Mori[7,15]. Given the favorable prognosis of the pure/predominant forms, coupled with the reported sensitivity to chemotherapy, some authors propose that radical cystectomy may be avoided in these cases and bladder-preserving strategy should further investigated[20].
While current standard neoadjuvant chemotherapy with MVAC (methotrexate, vincristine, adriamycin, cisplatin) or GC is the standard of care in muscle-invasive BC, only a minority of patients achieve CR and a majority of patients still relapse after radical cystectomy[21]. In addition, for patients who are cisplatin-ineligible, there is no standard of care for neoadjuvant therapy. Immunotherapy agents are being utilized in several ongoing and planned trials in this setting[22].
Recently, agents targeting the programmed death ligand 1 (PD-L1)/programmed death receptor 1 (PD-1) immune checkpoint were shown to display impressive antitumor activity in various solid or hematological malignancies, including a subset of previously treated and treatment-naïve patients with mUC. In heavily pre-treated UC, trials are suggesting objective response rates above 30%[23]. PD-L1 immunohistochemical expression is thought to represent a biomarker predictive of drug sensitivity[24]. Chang et al reported a high level of programmed cell death-ligand 1 (PD-L1) expression and infrequent driver mutations in PLELCs as compared with conventional non-small cell lung cancer (NSCLC)[24]. Other investigators also showed that a large proportion of patients with pulmonary LELC had positive expression of PD-L1, supporting the potential use of anti-PD-1/PD-L1-targeted therapies in this distinct type of NSCLC[25]. Interestingly, response to PD-1 directed therapy is seen even in patients with no evidence of PD-1 positivity on immunohistochemistry[23]. This has led to the development of enhanced biomarkers, including assessing DNA mutation rates and immune gene signatures to improve patient selection.
The tumor of our patient harbors a high TMB. High TMB has been shown to be associated with sensitivity to immune checkpoint inhibitors, including anti-CTLA-4 therapy in melanoma, and anti-PD-L1 therapy in urothelial carcinoma[26]. Increased TMB was associated with greater sensitivity to immunotherapeutic agents, including anti-CTLA-4, anti-PDL1, and anti-PD-L1 therapies. For patients with metastatic urothelial carcinoma, those who responded to atezolizumab treatment had a significantly increased mutational load [12.4 Mutations (mut) per Megabase (Mb)] compared to non responders (6.4 mut/Mb), and mutational load of 16 mut/Mb or higher was associated with significantly longer overall survival[27]. Therefore, increased TMB or PD-L1 evidence on IHC both serve as predictors for clinical response to targeted immunotherapy. This is especially useful in patients who do not qualify for traditional platinum-based therapy due to comorbidities.
Conclusion
To our knowledge, this is the first report in the literature describing the gene expression profiling of LELCB. Our case also highlights that the identification of very high mutation burden and multiple actionable genomic alterations in this patient’s tumor may serve as the potential targets for future immunotherapy and target therapy in this rare disease.
References
- 1. Singh, N.G., Mannan, A.A., Rifaat, A.A., et al. Lymphoepithelioma-like carcinoma of the urinary bladder: Report of a rare case. (2009) Ann Saudi Med 29(6): 478-481.
Pubmed || Crossref || Others - 2. Blasi, M.A., Ventura, L., Laguardia, M., et al. Lymphoepithelioma-like carcinoma involving the lacrimal gland and infiltrating the eyelids. (2011) Eur J Ophthalmol 21(3): 320-323.
Pubmed || Crossref || Others - 3. Brun, J.L., Randriambelomanana, J., Cherier, L., et al. Lymphoepithelioma-like carcinoma of the ovary: a case report and review of the literature. (2010) Int J Gynecol Pathol 29(5): 427-431.
Pubmed || Crossref || Others - 4. Mori, T., Sawada, M., Matsuo, S., et al. Lymphoepithelial-like carcinoma of the uterine cervix; a case report. (2011) Eur J GynaecolOncol 32(3): 325-327
Pubmed || Crossref || Others - 5. Gille, T.M., Miles, E.F., Mitchell, A.O. Lymphoepithelioma-like carcinoma of the skin treated with wide local excision and chemoradiation therapy: a case report and review of the literature. (2012) Case Rep Oncol Med 2012: 241816.
Pubmed || Crossref || Others - 6. Dinniwell, R., Hanna, W.M., Mashhour, M., et al. Lymphoepithelioma-like carcinoma of the breast: a diagnostic and therapeutic challenge. (2012) Current Oncology 19(3): 177-183.
Pubmed || Crossref || Others - 7. Lopez-Beltran, A., Luque, R.J., Vicioso, L., et al. Lymphoepithelioma-like carcinoma of the urinary bladder: a clinicopathologic study of 13 cases. (2001) Virchows Arch 438(6): 552-557.
Pubmed || Crossref || Others - 8. Holmäng, S., Borghede, G., Johansson, S.L. Bladder carcinoma with lymphoepithelioma-like differentiation: a report of 9 cases. (1998) J Urol 159(3): 779-782.
Pubmed || Crossref || Others - 9. Kozyrakis, D., Petraki, C., Prombonas, I., et al. Lymphoepithelioma-like bladder cancer: clinicopathologic study of six cases. (2011) Int J Urol 18(10): 731-734.
Pubmed || Crossref || Others - 10. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. (2014) Nature 507(7492): 315-322.
Pubmed || Crossref || Others - 11. Von Hoff, D.D., Stephenson, J.J. Jr, Rosen, P., et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. (2010) J Clin Oncol 28(33): 4877-4883.
Pubmed || Crossref || Others - 12. Bégin, L.R., Eskandari, J., Joncas, J., et al. Epstein-barr virus related lymphoepithelioma-like carcinoma of lung. (1987) J Surg Oncol 36(4): 280-283.
Pubmed || Crossref || Others - 13. Yang, A.W., Pooli, A., Lele, S.M., et al. Lymphoepithelioma-like, a variant of urothelial carcinoma of the urinary bladder: a case report and systematic review for optimal treatment modality for disease-free survival. (2017) BMC urology 17(1): 34.
Pubmed || Crossref || Others - 14. Izquierdo-García, F.M., García-Díez, F., Fernández, I., et al. Lymphoepithelioma-like carcinoma of the bladder: Three cases with clinicopathological and p53 protein expression study. (2004) Virchows Arch 444(5): 420-425.
Pubmed || Crossref || Others - 15. Mori, K., Ando, T., Nomura, T., et al. Lymphoepithelioma-like carcinoma of the bladder: A case report and review of the literature.(2013) Case Rep Urol: 3.
Pubmed || Crossref || Others - 16. Amin, M.B., Ro, J.Y., Lee, K.M., et al. Lymphoepithelioma-like carcinoma of the urinary bladder. (1994) Am J Surg Pathol 18: 466-473.
Pubmed || Crossref || Others - 17. Tamas, E.F., Nielsen, M.E., Schoenberg, M.P., et al. Lymphoepithelial-like carcinoma of the urinary tract: a clinicopathological study of 30 pure and mixed cases. (2007) Mod Pathol 20(8): 828-834.
Pubmed || Crossref || Others - 18. Serrano, G.B., Fúnez, F.A., López, R.G., et al. Bladder lymphoepithelioma-like carcinoma.Bibliographic review and case report. (2008) Arch Esp Urol 61(6): 723-729.
Pubmed || Crossref || Others - 19. Dinney, C.P, Ro, J.Y., Babaian, R.J., et al. Lymphoepithelioma of the bladder: A clinicopathological study of 3 cases. (1993) J Urol 149(4): 840-841.
Pubmed || Crossref || Others - 20. Pantelides, N.M., Ivaz, S.L., Falconer, A., et al. Lymphoepithelioma-like carcinoma of the urinary bladder: A case report and review of systemic treatment options. (2012) Urol Ann 4(1): 45-47.
Pubmed || Crossref || Others - 21. Dash, A., Pettus, J.A.T., Herr, H.W., et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. (2008) Cancer 113(9): 2471-2477.
Pubmed || Crossref || Others - 22. Balar, A.V., Galsky, M.D., Rosenberg, J.E., et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. (2017) Lancet 389(10064): 67-76.
Pubmed || Crossref || Others - 23. Bidnur, S., Savdie, R., Black, P.C. Inhibiting immune checkpoints for the treatment of bladder cancer. (2016) Bladder Cancer 2(1):15-25.
Pubmed || Crossref || Others - 24. Chang, Y.L., Yang, C.Y., Lin, M.W., et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. (2015) Lung Cancer 88(3): 254-259.
Pubmed || Crossref || Others - 25. Fang, W., Hong, S., Chen, N., et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. (2015) Onco target 6(32): 33019-330132.
Pubmed || Crossref || Others - 26. Chalmers, Z.R., Connelly, C.F., Fabrizio, D., et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. (2017) Genome Medicine 9(1): 34.
Pubmed || Crossref || Others - 27. Rosenberg, J.E., Hoffman-Censits, J., Powles, T., et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. (2016) Lancet 387(10031): 1909-1920.
Pubmed || Crossref || Others