Occurrence and Antimicrobial Susceptibility Profiling of Bacteria Isolated from Cultured Pangas Catfish (Pangasius pangasius) and Climbing Perch (Anabas testudineus) Fishes
Mohammad Zakerin Abedin1*, Md Babul Aktar1, SifatUz Zaman Md2, Rubait Hasan3, Laila Jarin4, Md. Rezaul Karim3, Md. Sadiqur Rahman5, Rabiul Islam5, MdEkhlas Uddin6*
Affiliation
1Dept. of Microbiology, KhwajaYunus Ali University, Bangladesh
2Dept. of Microbiology, Popular Diagnostic Centre Ltd, Bangladesh
3Dept. of Biochemistry and Biotechnology, KhwajaYunus Ali University, Bangladesh
4Dept. of Microbiology, LabAid Medical Centre Ltd, Bangladesh
5Dept. of Microbiology, Aqua Laboratory Quality Feeds limited, Bangladesh
6Dept. of Biochemistry and Molecular Biology, GonoBishwabidyalay, Bangladesh
Corresponding Author
Abedin, M.Z., Dept. of Microbiology, KhwajaYunus Ali University, Bangladesh; E-mail: zakerin.du2016@gmail.com
Citation
Abedin, M.Z., et al. Occurrence and Antimicrobial Susceptibility Profiling of Bacteria Isolated from Cultured Pangas Catfish (Pangasiuspangasius) and Climbing Perch (Anabas testudineus) Fishes. (2020) J Marine Biol Aquacult 6(1): 7-12.
Copy rights
© 2020 Abedin, M. Z. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Pangascatfish; Climbing perch; Bacterial pathogens; Antibiotic sensitivity profiles
Abstract
Aquaculture infections in fish are very common in Bangladesh. The aims and objectives of this study are to identify the pathogenic agents infecting aquaculture fish and sensitivity patterns of commonly used antimicrobial agents. A total of 20 infected fish samples were tested, among them pangas catfish (Pangasiuspangasius) and climbing perch koi (Anabas testudineus) fishes were 13 and 7 respectively. Cultural analysis was done by phenotypic examination along with biochemical examination to identify the isolates. Finally, antibiotic sensitivity was tested against conventionally used antibiotics. All the samples were aseptically collected from different Upazilla in Mymensingh region and tests were done in Quality Aqua Laboratory, Quality feeds Limited, Mymensingh between April 2019 to December 2019. The most predominant isolates in pangas fish were Flavobacteriumspp., (38.5%) and Edwarsiellaspp., (38.5%) and Aeromonasspp., (57.1%) in koi fishes. Like most of the previous reports, Flavobacteriumspp., Edwardsiellaspp., Aeromonasspp., was predominant, which corroborates this study. However the antimicrobial profile of the detected organisms differed compared to studies which were previously done. The isolates were mostly resistant to Amoxycillin. The pathogens showed remarkable amount of sensitivity against Ciprofloxacin, Cotrimoxazoleandand Doxycycline. The pathogens also showed moderate sensitivity to Erythromycin. For validating more reliability, this research needs further work.
Introduction
Fish is considered as one of the prime sources of food and income globally[1]. It is comparatively cheaper than other meat products; hence, it is more affordable to most people. Bangladesh is the world leading fish producing country. Common aquaculture practices in Bangladesh are mainly the culture of carps, tilapias, catfishes, climbing perches (koi), and shrimps, nile tilapia and pangas catfish are cultured mostly for commercial purpose by entrepreneurial farmers. Pangas catfish fish was first introduced to Bangladesh in 1989 from Thailand, aimed to upsurge overall aquaculture production and meeting the increasing demand for food fish[2].
Presently, this fish is considered as one of the important fishes in aquaculture of Bangladesh because of its fast growth, year-round production, and high productivity. The climbing perch fish (Anabas testudineus) is one of the important small indigenous spp., (SIS) fresh water fish of Bangladesh, which is locally known as koi fish. In Southeast Asian region, this fish is native and often found in fresh water sources of east India and south China[3]. But in Bangladesh, it is normally found in open water (streams, lakes, floodplain and beels), paddy fields, swamps and its habitats are heavily-vegetated and stagnant waters. Pathogenic micro organisms are a serious threat to fish production in all over the world due to high economic importance of diseases they cause. A number of bacterial pathogens have been found to cause diseases in fish worldwide. In fresh water fishes, bacteria were particular importance including Aeromonas spp., Pseudomonasspp., Streptococcusspp., Flavobacterium spp., Edwardsiella spp., Vibrio spp., and so on[4].
The bacterial infections which are caused by antibiotic-resistant bacteria is increasing all over the world. The main objective of this work was to isolate and identify bacteria from climbing perch (Anabas testudineus) and pangas catfish (Pangasiuspangasius) cultisupvated in the pond water of different areas in Mymensingh and to determine the level of antibiotic susceptibility rates of the isolated bacteria against seven antibiotics.
Materials and Methods
Study area and Design:
This cross section study involved collection of affected fish samples of climbing perch fish (Anabas testudineus) from 3Upazilla (Fulbaria, Muktagacha, Trishal) and pangas catfish (Pangasiuspangasius) from 4Upazilla (Fulbaria, Muktagacha, Trishal, Ishwarganj) of Mymensingh, Bangladesh. Bacteria were isolated from the collected fishes and identified by cultural and biochemical characteristics along with Gram staining technique. Antimicrobial susceptibility test was determined by the Kirby-Bauer disc diffusion method[5].
Figure 1: Map showing the geographical locations of districts sampled in this study
Sample collection and Transportation:
A total of 07 infected climbing perch fish (Anabas testudineus) and 13 infected pangas catfish (Pangasiuspangasius) samples were taken from different cultivated ponds between April 2019 to December 2019. During the collection, fish samples were maintained avoidance of touch and ice box were used to maintain cool chain. The samples were then brought to the laboratory of the Quality Aqua Laboratory, Quality feeds Limited, Mymensingh.

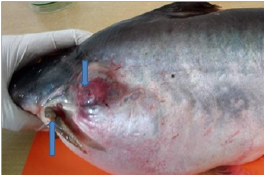
Figure 2: Infected fishes sampled from farmer’s ponds; (a) Koi (Anabas testudineus) with deep haemorrhagic ulcerative lesion on tail region; (b) Pangas (Pangasiuspangasius) with spoiled fin.
Processing of sample and enrichment of bacteria:
Aseptic measures were taken during the sampling procedure to prevent contamination. Three types of specimens of fishes were taken for analysis of microbiological test and the specimens are intestine, skin and gill. These specimens were taken on a sterile chopping board and then minced and grinded together. Ten (10) gm of samples were homogenized with 90 milliliters (ml) of freshly prepared 0.1% peptone water and then 0.1 ml of homogenized sample was inoculated according to standard methods on to both selective media and conventional media such as RimlerShotts Medium Base agar (for Aeromonasspp.), Pseudomonas Baseagar (for Pseudomonas spp.), Thiosulfate citrate bile salt sucrose (TCBS) agar (for Vibrio spp.), Tryptic Soy Agar for enrichment of bacterial isolates, Blood agar, and MacConkey agar. Then all media were incubated at 37ºC for 24 hours.
Identification of Bacterial Pathogens
Morphological Characterization:
The suspected bacterial colonies obtained from different culture plates were isolated and then streaked on TSA slants, MIU medium, Simon citrate agar slant and then incubated overnight at 37ºC. For morphological characterization,colonial characteristics, and bacterial cell morphology such as size, shape and so on.
Biochemical Characterization:
Various biochemical tests such as alkaline and acidic reaction, H2S (hydrogen sulfide) production, gas production, MIU (motility , indole, urease), oxidase, catalase, Methyl Red (MR) test, and Voges-Proskaure (VP) test were performed to characterize the pathogens. The biochemical tests were done to identify the pathogens following Bergey’s manual of Bacteriological classification[6].
Gram Staining Method:
These methods were done to distinguish between Gram positive and Gram negative bacteria. This technique was done on a clean grease free glass slide from a single bacterial colony by crystal violet solution (1 min), iodine solution (1 min), and safranin (2 min). Then the slide was washed properly by tap water before starting the next step. The slide image analysis was performed using a light microscope (Nikon Co., Tokyo, Japan) at 10X100 with immersion oil.
Antimicrobial susceptibility test:
The Kirby- Bauer disc diffusion methods (CLSI, 2015)[7] were performed for antibiogram patterns of all the pathogenic bacteria which were isolated. In this study, the commonly used antibiotics were included: Amoxicillin (10μg), Ciprofloxacin (5μg), Colistin (25μg), Clotetracyclin (30μg), Doxycycline(30μg), Erythromycin (15μg) and Co-trimoxazole (25μg). Aeromonashydrophila (ATCC 7966), Pseudomonas aeruginosa (ATCC 27853), Flavobacteriumcolumnare (ATCC 23463), Edwardshiellatarda (ATCC 15947) were used as standard throughout the study for culture and antimicrobial susceptibility testing.
The suspected isolated colonies of bacteria were taken to sterile PBS (phosphate buffered saline) water and then adjusted to 0.5 McFarland’s turbidity standard. The bacterial suspension was spread onto Mueller–Hinton agar (Himedia, India) and then antibiotic discs (Himedia, India) were placed and incubated at 37ºC for 24 hours. Around the discs, the inhibition of antibiotic zone was estimated in diameter of millimeter (mm). The zone of inhibition was scaled from the focal point of the anti-microbial plate as far as possible of the reasonable zone where microscopic organisms could be seen developing. The interpretation of antibiogram was measured in millimeter (mm) of diameters as resistance, sensitive and intermediate as per the producer’s guidelines.
Statistical Analyses of Experimental Data:
Data obtained were analyzed by SPSS version 20 and Excel 2016. Descriptive statistics and chi-square tests were done to check the statistical evaluation. The p-value that considered significant was <0.5.
Results
Occurrence of bacteria in Pangas catfish (Pangasiuspangasius)
The bacteria isolated were Aeromonas spp., Edwarsiella spp., Flavobacteriumspp., and Streptococcus species from 13 infected pangas catfish samples which were identified by using morphological properties, Gram staining, and series of biochemical tests. Edwardsiella spp., 5(38.5%) and Flavobacterium spp., 5(38.46%) were the most dominant species among the isolates. The rest of 2(15.4%) Aeromonas spp., and 1(7.6%) Streptococcus spp.,were confirmed from pangas catfishes as indicated in the Figure 3.
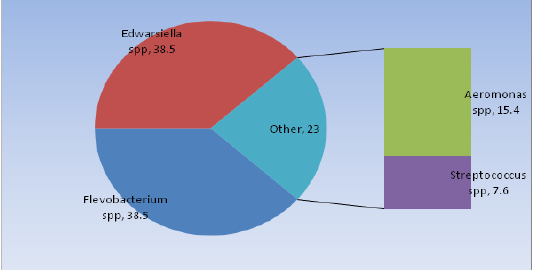
Figure 3: Prevalence of the isolated bacteria in Pangas fish.
Occurrence of Bacteria in Climbing Perch Fish (Anabas testudineus)
The bacteria isolated were Aeromonas spp., Streptococcus spp.,and Pseudomonas species from 07 infected climbing perch fish (Anabas testudineus) commonly known as koifish samples which were identified using morphological properties, Gram staining, and series of biochemical tests. Aeromonas spp., 4(57.14%) was the most dominant isolates and rest of 2(28.6%) Streptococcus spp., and 1(14.3%) Psudomonas species from fish samples as indicated in Figure 4.
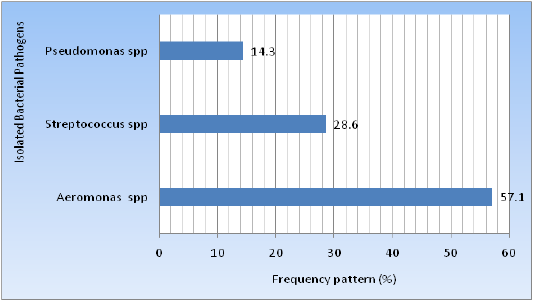
Figure 4: Prevalence of the isolated bacteria in climbing perch fish.
Biochemical tests for Bacterial Identification
The identification of pure bacterial isolates were done by biochemical characteristics which were alkaline reaction, acidic reaction, H2S (hydrogen sulfide) production, Gas production, Motility test, indole production, urea hydrolysis, catalase test, oxidase test, Methyl -Red (MR) test, Voges- Proskaure (VP) test are presented in Table 1.
Table 1: Result of biochemical tests along with gram staining of the isolated bacterial spp., from infected fishes.
|
|
|
KIA |
MIU medium |
|
|
|
|
|
|||||
|
Bacterial isolates |
Gram reaction |
Slant |
Butt |
Gas |
H2S |
Mot |
Indole |
Urea |
Oxidase |
S. citrate |
MR |
VP |
Cat |
|
Aeromonasspp |
Gram negative |
R |
Y |
+ |
- |
+ |
+ |
- |
+ |
± |
+ |
± |
+ |
|
Pseudomonas spp |
Gram negative |
R |
R |
- |
- |
+ |
- |
± |
+ |
+ |
- |
- |
+ |
|
Streptococcus spp |
Gram positive |
Y |
Y |
- |
- |
+ |
- |
+ |
- |
+ |
+ |
+ |
+ |
|
Flavobacteriumspp |
Gram negative |
Y |
W |
- |
- |
+ |
- |
- |
+ |
+ |
+ |
- |
+ |
|
Edwardsiellaspp |
Gram negative |
R |
Y |
+ |
+ |
+ |
+ |
- |
- |
- |
+ |
- |
+ |
(+) = Positive; (-) = Negative reaction; (±) = Variable; R = Red (Alkaline reaction); Y=Yellow (Acid reaction); W = Week positive, H2S = Hydrogen sulphide (Blackening); MR = Methyl Red; VP = Voges-Proskaure; KIA = Kligler Iron agar; MIU = Motility indole urea test; Cat=Catalase test; and Mot=Motility test.
Overall Antimicrobial Susceptibility of Pangas Catfish:
In this experiment, all of the isolated pathogenic bacteria showed 13/13(100%) resistant to Amoxicillin and Erythromycin. All the strains showed sensitive to Enorfloxacin, Ciprofloxacin, Doxycycline, Cotrimoxazole and Colistinwere 12/13(92.3%), 12/13(92.3%), 11/13(84.6%), 12/13(92.3%) and 8/13(61.5%) respectively. Colistin and Chlortetracycline showed intermediate sensitivity such as 5/13(38.5%) and 2/13(15.4%) respectively (Table 2 and Figure 5).
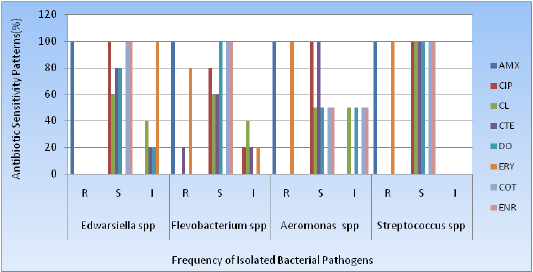
Figure 5: Antimicrobial profiling of catfish pathogens; R: Resistant; S: Sensitive; I: Intermediate sensitive;AMX: Amoxycillin; CIP: Ciprofloxacin; CL: Colistin; CT: Chlortetracycline; DO: Doxycyclline; ERT: Erythromycin; COT:Cotrimoxazole; ENR: Enorfloxacin.
Overall Antimicrobial Susceptibility Patterns of Climbing PerchFish:
In this experiment, all of the isolated pathogenic bacteria showed 7/7(100%) resistant to Amoxicillin and 3/7 (42.9%) resistant to Chlortetracycline. All the strains showed sensitive to Ciprofloxacin, Doxycycline, Cotrimoxazoleand Colistinwere 6/7(85.7%), 4/7(57.1%), 6/7(85.7%) and 2/7(28.6%) respectively. Erythromycin and Colistin showed intermediate sensitivity such as 5/7(71.4%) and 2/7(28.6%) respectively (Table 3 and Figure 6).
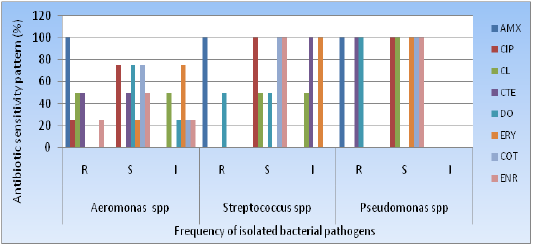
Figure 6: Antimicrobial profiling of climbing perch fish pathogens;R: Resistant; S: Sensitive; I: Intermediate sensitive;AMX: Amoxycillin; CIP: Ciprofloxacin; CL: Colistin; CT: Clotetracyclin; DO: Doxycillin; ERT: Erythomycin; COT:Cot-trimoxyzole; ENR: Enrofloxacin.
Table 2: Antibiotic sensitivity patterns all of the individual isolates.
|
Bacterial isolates |
Sensitivity pattern |
AMX |
CIP |
CL |
CTE |
DO |
ERY |
COT |
ENR |
|
Edwardsiella spp (n=05) |
R |
100 |
0 |
0 |
0 |
0 |
100 |
0 |
0 |
|
S |
0 |
100 |
60 |
80 |
80 |
0 |
100 |
100 |
|
|
I |
0 |
0 |
40 |
20 |
20 |
0 |
0 |
0 |
|
|
Flevobacterium spp (n=05) |
R |
100 |
0 |
0 |
20 |
0 |
80 |
0 |
0 |
|
S |
0 |
80 |
60 |
60 |
100 |
0 |
100 |
100 |
|
|
I |
0 |
20 |
40 |
20 |
0 |
20 |
0 |
0 |
|
|
Aeromonas spp (n=02) |
R |
100 |
0 |
0 |
0 |
0 |
100 |
0 |
0 |
|
S |
0 |
100 |
50 |
100 |
50 |
0 |
50 |
50 |
|
|
I |
0 |
0 |
50 |
|
50 |
0 |
50 |
50 |
|
|
Streptococcus spp (n=01) |
R |
100 |
0 |
0 |
0 |
0 |
100 |
0 |
0 |
|
S |
0 |
100 |
100 |
100 |
100 |
0 |
100 |
100 |
|
|
I |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
N: number; R: Resistant; S: Sensitive; I: Intermediate sensitive; AMX: Amoxycillin; CIP: Ciprofloxacin; CL: Colistin; CT: Chlortetracycline; DO: Doxycyclline; ERT: Erythromycin; COT:Cotrimoxazole; and ENR: Enorfloxacin.
Table 3: Antibiotic sensitivity patterns all of the individual isolates.
|
Bacterial isolates |
Sensitivity pattern |
AMX |
CIP |
CL |
CTE |
DO |
ERY |
COT |
ENR |
|
Aeromonas spp (n=04) |
R |
100 |
25 |
50 |
50 |
0 |
0 |
0 |
25 |
|
S |
0 |
75 |
0 |
50 |
75 |
25 |
75 |
50 |
|
|
I |
0 |
0 |
50 |
0 |
25 |
75 |
25 |
25 |
|
|
Streptococcus spp (n=02) |
R |
100 |
0 |
0 |
0 |
50 |
0 |
0 |
0 |
|
S |
0 |
100 |
50 |
0 |
50 |
0 |
100 |
100 |
|
|
I |
0 |
0 |
50 |
100 |
0 |
100 |
0 |
0 |
|
|
Pseudomonas spp (n=01) |
R |
100 |
0 |
0 |
100 |
100 |
0 |
0 |
0 |
|
S |
0 |
100 |
100 |
0 |
0 |
100 |
100 |
100 |
|
|
I |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
N: number; R: Resistant; S: Sensitive; I: Intermediate sensitive;AMX: Amoxycillin; CIP: Ciprofloxacin; CL: Colistin; CT: Chlortetracycline; DO: Doxycyclline; ERT: Erythromycin; COT:Cotrimoxazole; and ENR: Enorfloxacin.
Discussion
Fish farming is such an important sector which has been contributing tremendously to our economy. It is one of the most promising industries with the brightest future for our country. Though this sector has such potentiality, fish farming is confronted with acute problem of disease like bacterial, fungal, viral disease and also skin ulcer which can be caused by different factor. Those factors can cause great harm to the production cycle. Bacterial agents are responsible mostly for skin ulcer. In both species of pangas and climbing perch fishes,the most predominant pathogens were Aeromonasspp., Edwardsiella spp., and Flavobacteriumspp., similar findings have been also reported in Mohantyet al[4] and Hemstreet[8].
Pangas (Pangasiuspangasius) is the most frequently available fishes in Bangladesh. In this study, pond water cultivated pangas fishes were infected with several types of bacterial pathogens. The most isolated bacteria were Flavobacteriumspp., 5(38.5%) and Edwarsiella spp., 5(38.5%) followed by Aeromonasspp., 2(15.4%) and Streptococcusspp., 1(7.7). In our study, all bacterial isolates of Edwarsiellaspp., showed 100% sensitive to Co-trimoxazole and Ciprofloxacin; Flavobacteriumspp., were 100% sensitive to Doxycycline, Co-trimoxazole and Enorfloxacin; Aeromonasspp., were 100% sensitive to Clotetracyclin, Ciprofloxacin and only one strain of Streptococcus spp., was 100% sensitive to Doxycycline, Co-trimoxazole,Ciproloxacin. Clotetracycline, Amoxycillin. On the other hand, all organisms showed high degree of resistance to Erythromycin.
Climbing Perch fish (Anabas testudineus) is the most available and cheap fish in Bangladesh. In our research, Climbing Perch fish cultivated in pond water were infected by bacterial pathogens of Aeromonas spp., 4(57.1%), Streptococcus spp., 2(28.6%) and Pseudomonas spp., 1(14.3%). This type of comparable isolation rate was also reported by Shittuet al[9]. In our investigation, Aeromonas spp., was the largest isolated bacterial pathogens and the highest sensitive to 75% Co-trimoxazole, Doxycycline and Ciprofloxacin each.
The second largest isolates were Streptococcus spp., which was 100% sensitive to Ciprofloxacin, Co-trimoxazole and Enorfloxacin; third one of Pseudomonas spp., was 100% sensitive to Ciprofloxacin, Colistin, Erythromycin, Co-trimoxazole and Enorfloxacin. Streptococcus spp., was 100% resistant to Amoxycillin and Pseudomonas spp., showed 100% resistant to Amoxycillin, Chlortetracycline and Doxycycline. The overall study showed that Amoxycillin was the most resistant antibiotic against all bacterial isolates. The significant level of resistance from regularly utilized antibiotics is similar with Hussainet al[10] and Mostafaet al[11]. Further research is needed to better understand the real situation of aquaculture fish infection and treatment efficacy in greater Mymensingh.
Conclusion
This study intended to ascertain the existing situation of aquaculture infection and drug resistance among Pangas and climbing perch fish. From this study, it has been concluded that Flavobacterium spp., Edwarsiella spp., and Aeromonas spp., were the predominant aquaculture infection followed by Streptococcusspp., and Pseudomonasspp., Ciprofloxacin, Cotrimoxazole and Doxycycline were found as a reliable therapeutic intervention for the investigated pathogens because of their broad spectrum activity in the current study. Antibiotic selection should be guided by culture and sensitivity test and empirical drug must be decided on the recent antibiogram of a particular geographical area.
Acknowledgements
The authors would like to thank Department of Microbiology, School of Biomedical Science, Khwaja Yunus Ali University, Sirajganj and Aqua Laboratory Quality Feeds Limited Mymensingh, Bangladesh for assistance this research.
Irreconcilable situations Statement
The authors announce that there is no irreconcilable situation with respect to the publication of this article.
Monetary Disclosure Statements
This investigation was without supported
Animal Rights Statement
No animal experiments were conducted.
References
- 1. Tacon, A.G.J., Metian M. Fish matters: importance of aquatic foods in human nutrition and global food supply. (2013) Rev Fish Sci21(1): 22-38.
- 2. Exotic Fish. Banglapedia. (2015) National Encyclopedia of Bangladesh.
PubMed│CrossRef│Others
- 3. Chakraborty, B.K., Haque, S.M. Growth yields and returns to Koi, Anabas testudineus (Bloch, 1792) under semi intensive agriculture system using different seed types in Bangladesh.(2014) J Fisheries Livest Prod2: 113-119.
- 4. Mohanty, B.R., Sahoo, P.K. Edwardsiellosis in fish: A brief review. (2007)J Biosci32(7): 1331-1344.
- 5. Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. (2009) American Society for Microbiology.
PubMed│CrossRef│Others
- 6. Abedin, M.Z., Rahman, M.S., Hasan, R., et al. Isolation, identification, and antimicrobial profiling of bacteria from aquaculture fishes in pond water of Bangladesh. (2020) Am J Pure ApplSci2(3): 39-50.
- 7. CLSI - Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement. (2015) Wayne, PA, USA. CLSI.
PubMed│CrossRef│Others
- 8. Hemstreet, B. An update on Aeromonashydrophila from a fish health specialist for summer. (2010) Catfish Journal24: 4.
PubMed│CrossRef│Others
- 9. Shittu, A.O., Nubel, U., Udo, E.E., et al. Characterization of methicillin-resistant Staphylococcus aureus (MRSA) isolates from hospitals in KwaZulu-Natal (KZN) province, Republic of South Africa. (2009) J. Med. Microbiol58: 1219-1226.
- 10. Hussain, M.G. (2014) Global opportunity for fisheries and aquaculture in Bangladesh. Paper presented in the 2nd International Exhibition and Seminar Dairy, Aqua and Pet Animals-2014, Dhaka, Bangladesh.
PubMed│CrossRef│Others
- 11. Mostafa, M., Ahamed, F. Pethogenesis of Aeromonashydrophilaon Heteropneustesfossilis. (2008) Bangladesh J of Fis8: 38-41.
PubMed│CrossRef│Others












