Pegfilgrastim Administration Timing and its Effect on Febrile Neutropenia in Pediatric Cancer Patients
Joana M. Mack1,2*, Beverly J. Spray3, Delanie Mack, B.S.4, Katherine Mason1,2, David Becton1,2, Kevin Bielamowicz1,2
Affiliation
1Department of Pediatrics, Division of Pediatric Hematology-Oncology, University of Arkansas for Medical Sciences
2Arkansas Children’s Hospital
3Arkansas Children’s Research Institute
4University of Arkansas, Fayetteville
Corresponding Author
Joana M. Mack, Department of Pediatrics, Division of Pediatric Hematology-Oncology, University of Arkansas for Medical Sciences, 1 Children’s Way, Slot 512-10A, Tel: (501) 364-1494/ Fax: (501) 364-4332; E-mail: jmmack@uams.edu
Citation
Mack, J.M., et al. Pegfilgrastim Administration Timing and its Effect on Febrile Neutropenia in Pediatric Cancer Patients. (2019) Intl J Cancer Oncol 6(2): 37-41.
Copy rights
© 2019 Mack, J.M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Pegfilgrastim; Febrile neutropenia; Pediatric cancer
Abstract
Background: Pegfilgrastim (Peg-GCSF) is administered at the completion of chemotherapy to shorten the time that patients are neutropenic. Several adult studies reveal a higher risk of febrile neutropenia among patients who received Peg-GCSF within 24 hours of completing chemotherapy compared to those who received growth factor on days 2-4 after chemotherapy, while other studies show no increased risk or are inconclusive. Currently, there are no data in pediatric malignancies that evaluate the effect of timing of Peg-GCSF administration on the rates of febrile neutropenia.
Objective: To determine if there was a difference in the incidence of febrile neutropenia when Peg-GCSF was administered within 24 hours or greater than 24 hours after completion of chemotherapy.
Methods: An IRB-approved retrospective study was conducted at Arkansas Children’s Hospital. Medical records of patients who received Peg-GCSF after chemotherapy from 2010-2017 were analyzed. Eligible patients were those with a diagnosis of malignancy other than leukemia who had at least one dose of Peg-GCSF after chemotherapy as part of first-line treatment, and had available data on the timing of Peg-GCSF administration in relation to chemotherapy administration.
Results: A total of 1,458 doses of Peg-GCSF given to 237 patients were evaluated. The frequency of febrile neutropenia was 24.6% for less than 24 hours and 25.0% for more than 24 hours administration of Peg-GCSF.
Conclusion: There was no statistically significant difference in the frequency of febrile neutropenia among patients whether Peg-GCSF was given prior to or after 24 hours after the completion of chemotherapy.
Introduction
Neutropenia is a common side effect of myelosuppressive chemotherapy, often causing hospitalization to manage febrile patient who have an increased risk of sepsis and other serious infections. Granulocyte-colony stimulating factors (G-CSF) are administered to shorten the duration of neutropenia[1,2]. Peg-GCSF, a pegylated form of G-CSF, has a half-life between 15-80 hours and therefore requires only one dose per chemotherapy course[3,4].
The Federal Drug Administration (FDA) approval guidelines suggest delaying Peg-GCSF dosing for at least 24 hours after the completion of each chemotherapy cycle[5] to minimize the risk of exposing stimulated myeloid precursor cells to the toxic effects of chemotherapy which could prolong the duration of neutropenia[6,7]. The European Organization for Research and Treatment of Cancer (EORTC) 2010 evidence based guidelines also recommended that primary prophylaxis with G-CSF should be given 24-72 hours after the end of chemotherapy[8]. However, there is variation in practice at some centers, and many patients have received Peg-GCSF within 24 hours of completing their chemotherapy cycle as shown in retrospective studies[9].
Patients receiving Peg-GCSF 24 hours after the completion of chemotherapy as dictated by the FDA indications have to return to an outpatient clinic or infusion center to receive Peg-GCSF, stay in the hospital for an additional 24 hours even if post-chemotherapy fluids are not indicated, or have home health services arranged for home delivery and/or administration of Peg-GCSF. This could potentially cause several challenges for patient care, including the inconvenience and cost of an additional trip to an outpatient clinic or extended inpatient stay, additional time for caregivers away from work or home, increased exposure to sick contacts, and anxiety associated with home administration. Adult data regarding the timing of Peg-GCSF administration and its effects on fever and neutropenia in cancer patients receiving myelosuppressive chemotherapy is not conclusive. A prospective study in adults with breast cancer and lymphoma recommended administration of Peg-GCSF 24 hours after completion of chemotherapy due to longer and more severe neutropenia when administered the same-day of completion[6]. Additionally, an adult study evaluating patients with intermediate/high-risk regimens for solid tumors revealed a higher risk of febrile neutropenia among patients who received Peg-GCSF the same day of chemotherapy compared to those who received growth factor on days 2-4 from chemotherapy[9]. However, a retrospective study evaluating patients with gynecological malignancies did not demonstrate increased toxicity in same day versus next day administration of Peg-GCSF[3].
There are no data in pediatric malignancies that evaluate whether timing of Peg-GCSF administration leads to different rates of febrile neutropenia. The primary objective of this retrospective study was to determine if there was a difference in incidence of febrile neutropenia when pegfilgrastim was administered the same day versus the next day after completion of chemotherapy. Secondary objectives aimed to describe practice among pediatric hematology/oncology providers regarding timing of Peg-GCSF administration.
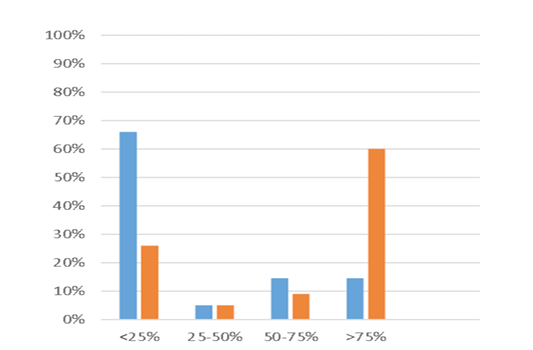
Figure 1: Provider Timing of Administration of Peg-GCSF. Y-axis represents the percentage of provider responses for each category. X-axis represents the frequency at which individual providers administered Peg-GCSF before or after 24 hours from the completion of chemotherapy. Blue bar – 0-24 hours after the completion of chemotherapy. Orange bar – 24-72 hours after the completion of chemotherapy.
Methods
Data Search
The medical records of all patients who received Peg-GCSF after chemotherapy for malignancy at Arkansas Children’s Hospital between 2010 and 2017 were retrospectively reviewed. This study was approved by the institutional review board at our institution. Eligible patients for this analysis were those who had at least one dose of Peg-GCSF after first-line chemotherapy for any malignancy other than leukemia, and for whom there was available data on the timing of Peg-GCSF administration. 2,011 doses of Peg-GCSF were administered to 377 patients. Doses excluded for analysis included 49 doses administered to 10 patients who did not have data available regarding the timing of Peg-GCSF administration, 42 doses given to 9 patients with leukemia, and 462 doses given to 164 patients for treatment of relapsed or progressive disease after first-line therapy. A total of 1458 doses of Peg-GCSF given to 237 patients were included in the analysis. Additional data were collected from the medical record and included age, sex, ethnicity, diagnosis, admissions for fever and severe neutropenia, blood culture results, neutrophil counts, and chemotherapy medications. Fever was defined as a temperature of ≥ 38.4℃. Severe neutropenia was defined as ≤ 500 cells/µL.
Survey
An anonymous survey was sent to pediatric hematology/oncology providers (physicians, advanced practitioners, and hematology/oncology fellows) through a national organization list serve to evaluate their practice regarding timing and setting of administration of Peg-GCSF. Providers were asked if they administered Peg-GCSF less than 24 hours or 24-72 hours from the completion of chemotherapy and why they chose one or the other. They were also asked to give their opinion on the efficacy of Peg-GCSF when delivered less than 24 hours or 24-72 hours from completion of chemotherapy.
Statistics
Comparisons of patient characteristics between timing of Peg-GCSF administration (i.e., same day or next day) included t-tests for continuous data and chi-square tests for categorical data. To determine if the primary outcome, frequency of febrile neutropenia, was associated with timing of the administration of Peg-GCSF, a general estimating equation (GEE) was employed. Data from multiple chemotherapy sessions per patient were included in the GEE model to account for the correlation structure of repeated measures[10]. The GENMOD procedure in SAS version 9.4 was employed to invoke the GEE method[11]. This procedure incorporated a GEE logistic regression analysis and employed the logit link and REPEATED statement that specified an exchangeable correlation structure of repeated measures[10]. Demographic variables were initially tested in the GEE model. Only age had a significant effect on neutropenia and continued as a covariate in the model.
Secondary analyses included the evaluation of the effect of timing of Peg-GCSF administration on neutropenia by type of cancer and chemotherapy. The same GEE method was employed. Each sub-analysis (i.e., cancer and chemotherapy) was considered a separate family of hypotheses; thereby, p-values associated with multiple testing within each family were adjusted to control for the false discovery rate (FDR)[12]. Holm’s sequential Bonferroni method was used to correct for multiple testing[13].
Results
Table 1 presents the patient characteristics based on the timing of Peg-GCSF administration. Mean age was 9.6 years for patients in the less than 24 hours administration group and 9.8 years for patients in the 24-72 hours group (range 1 month to 22 years) and did not differ significantly between administration times. While there was a greater percentage of males and fewer females in the more than 24 hours “next day” administration of Peg-GCSF compared to the less than 24 hours administration, this difference was not statistically significance at the 0.05 level. The mean number of chemotherapy cycles was five and did not differ significantly between administration times. The frequency of the predominant cancer types also did not differ between administration times of Peg-GCSF. The predominant chemotherapy medications were cyclophosphamide (n = 797 cycles), etoposide (n = 736), doxorubicin (n=520), ifosfamide (n = 456), cisplatin (n = 330), and carboplatin (n = 67) given in combination with each other; however, we evaluated them separately to determine if the prevalence of each medication was similar across Peg-GCSF administration time. Table 1 displays the frequencies and associated P-values for each medication. No statistical differences were found to exist between same day and next day administration for any chemotherapy medication.
Table 1: Patient Characteristics by Timing of Administration of Pegfilgrastim
|
Characteristics |
Same day (N=190) |
Next day (N=47) |
P-value |
|
Age, mean (SD) |
9.6 (5.9) |
9.8 (6.2) |
0.805 |
|
Gender, N (%) |
|
|
|
|
Female |
93 (49) |
16 (34) |
0.067 |
|
Male |
97 (51) |
31 (66) |
|
|
Chemo cycles, mean (SD) |
5.0 (3.9) |
4.9 (4.7) |
0.903 |
|
Pegfilgrastim doses |
1242 |
216 |
|
|
Cancer type, N (%)a |
|||
|
Ewing’s Sarcoma |
1 (9.5) |
7 (22.6) |
0.092 |
|
Hodgkin Lymphoma |
37 (31.9) |
7 (22.6) |
|
|
Medulloblastoma |
13 (11.2) |
5 (16.1) |
|
|
Neuroblastoma |
17 (14.7) |
7 (22.6) |
|
|
Osteosarcoma |
16 (13.8) |
4 (12.9) |
|
|
Rhabdomyosarcoma |
22 (18.9) |
1 (3.2) |
|
|
Chemotherapy, N (%)b |
|||
|
Cyclophosphamide |
124 (65.3) |
27 (57.5) |
0.318 |
|
Ifosfamide |
11 (5.8) |
5 (10.6) |
0.236 |
|
Cisplatin |
62 (32.6) |
17 (36.2) |
0.645 |
|
Carboplatin |
17 (9.0) |
6 (12.8) |
0.429 |
|
Etoposide |
86 (45.3) |
21 (44.7) |
0.943 |
|
Doxorubicin |
86 (45.3) |
23 (48.9) |
0.651 |
aCancer type refers to the most prevalent cancers in the sample and will not sum to the total sample size due to other less frequent cancers found in the sample.
bSeparate analyses were performed for each chemo medication to ensure independent observations due to multiple combinations of chemotherapy per patient.
The frequency of febrile neutropenia for all cycles was 24.6% and 25.0% for same day and next day administration of Peg-GCSF, respectively. The results of the GEE logistic regression analyses of febrile neutropenia are presented in Table 2. After adjustment for age and taking into account the correlated nature of multiple cycles per patient, febrile neutropenia occurrence did not differ significantly between administration times of Peg-GCSF (p = 0.708).
Table 2: Results of the GEE Logistic Regression Analyses of Febrile Neutropenia
|
|
Febrile Neutropenia N (%) |
Adjusted Ora (95% CI) |
P-valueb |
|
|
Population |
Same day |
Next day |
||
|
Entire sample |
306 (24.6) |
54 (25.0) |
0.93 (0.62-1.39) |
0.708 |
|
Cancer type |
|
|
|
|
|
Ewing’s Sarcoma |
39 (21.7) |
11 (22.0) |
1.29 (0.70-2.40) |
0.417 |
|
Hodgkin Lymphoma |
28 (17.6) |
6 (26.1) |
0.66 (0.18-2.42) |
0.53 |
|
Medulloblastoma |
52 (59.1) |
6 (25.0) |
0.23 (0.06-0.87) |
0.03 |
|
Neuroblastoma |
27 (28.7) |
9 (45.0) |
0.48 (0.19-1.26) |
0.136 |
|
Osteosarcoma |
26 (28.9) |
5 (20.0) |
0.72 (0.24-2.11) |
0.546 |
|
Rhabdomyosarcoma |
44 (19.6) |
1 (9.1) |
0.95 (0.48-2.39) |
0.194 |
|
Chemotherapy, N (%) |
||||
|
Cyclophosphamide |
196 (28.8) |
36 (30.8) |
0.85 (0.51-1.43) |
0.539 |
|
Ifosfamide |
84 (21.7) |
10 (14.7) |
2.55 (0.99-6.55) |
0.053 |
|
Cisplatin |
98 (36.4) |
25 (41.0) |
0.62 (0.32-1.20) |
0.159 |
|
Carboplatinc |
11 (19.3) |
0 (0) |
- |
- |
|
Etoposide |
133 (22.0) |
25 (19.4) |
1.32 (0.72-2.40) |
0.372 |
|
Doxorubicin |
98 (21.7) |
20 (29.4) |
1.34 (0.79-2.24) |
0.275 |
N refers to the total number of cycles across all patients. N for same day is 1242 and for next day if 216.
a Odds ratios (OR) were adjusted for age of the patient at the time of chemotherapy.
b Raw P-values, when significant, were corrected for multiple testing within cancer type and chemotherapy medication using the Holm-Bonferroni formula.
cAnalysis could not be completed due to the iteration limit was exceeded.
Secondary analyses were conducted to determine if neutropenia differed by administration time within the more prevalent cancer diagnoses. There was no significant difference in the frequency of febrile neutropenia between same day and next day administration of Peg-GCSF for each cancer type except medullobastoma, as more patients with medulloblastoma developed febrile neutropenia when given Peg-GCSF less than 24 hours after completing chemotherapy compared to later. However, after correcting for multiple testing, this difference was no longer significant at the adjusted significance level of 0.008. Additionally, analyses of the frequency of febrile neutropenia was conducted for each of the most common chemotherapy medications regardless of the combination of medications. The difference in the incidence of febrile neutropenia between same day and next day administration approached significance at P=.053 for ifosfamide. There was a greater percentage of neutropenia in the same day group (21.7%) compared to the next day group (14.7%). However, a correction for multiple testing rendered this result nonsignificant at the adjusted level of significance of .01.
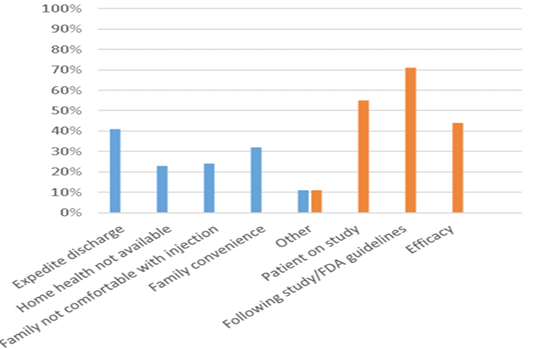
Sixty providers from throughout the United States and Canada completed the survey. Sixty percent of providers administered Peg-GCSF more than 75% of the time at 24-72 hours after the completion of chemotherapy. Only 14% of providers administered Peg-GCSF more than 75% of the time within 24 hours of completing chemotherapy. Peg-GCSF was administered most often in clinic, by home health providers, or by parents at home. Seventy percent of providers did not feel that the efficacy of Peg-GCSF was decreased if administered prior to 24 hours after the completion of chemotherapy. Fifty-three percent of providers did not feel the efficacy of Peg-GCSF was improved if administered 24-72 hours from the completion of chemotherapy.
Discussion
Febrile neutropenia following myelosuppressive chemotherapy can lead to prolonged hospital stays, bacteremia, higher hospital costs, and mortality. Administration of Peg-GCSF can minimize such events. This is the first study in pediatric cancer patients to evaluate the incidence of febrile neutropenia in relation to timing of pegfilgrastim administration. Our study shows that there is no statistically significant difference between rates of febrile neutropenia in pediatric malignancies when Peg-GCSF is given within 24 hours of the completion of chemotherapy compared to more than 24 hours after.
Figure 2: Situations for administering Peg-GCSF within 24 hours or 24-72 hours after the completion of chemotherapy. Y-axis represents the percentage of responses for each category. X-axis represents the reasons for administration before or after 24 hours from the completion of chemotherapy. Blue bar – 0-24 hours after the completion of chemotherapy. Orange bar – 24-72 hours after the completion of chemotherapy.
The overall rate of febrile neutropenia is comparable to other data with the use of Peg-GCSF in pediatric malignancies. Borinstein et al published a study in which growth factor support was administered to patients no earlier than 24 hours following the completion of chemotherapy[4]. This study evaluated 47 pediatric patients with solid tumors who received Peg-GCSF following myelosuppressive chemotherapy. The incidence of febrile neutropenia was comparable to patients who received filgrastim, a short acting G-CSF. All doses of Peg-GCSF were administered 24-48 hours after completion of chemotherapy. The overall incidence of febrile neutropenia was 28.0%, while the overall rate in our study was 24.7%[4].
There are several limitations to our study. This is a single institutional study and based on the provider survey results, our institution’s practice falls within the minority of pediatric oncology providers by giving Peg-GCSF less than 24 hours from the completion of chemotherapy most of the time. Out of 1,458 observations, 1,242 (85%) administrations of Peg-GCSF were given less than 24 hours from the completion of chemotherapy. More balanced numbers between these two groups would be ideal, but the number of events was large enough to allow for appropriate analysis. While this needs to be taken into account, it also shows the great potential for impacting patient care if the practice of the majority of pediatric oncology providers could be influenced to allow for better convenience and cost for patients.
Additionally, this was a non-randomized, retrospective study which relied on several factors such as provider preference, convenience for families, and participation in clinical trials to decide the timing of Peg-GCSF administration for each patient, which could have influenced outcomes. A prospective, randomized trial is needed to confirm this data.
Several different types of malignancies and chemotherapy regimens were included in this study. With smaller numbers of each cancer type, we had limited power to determine the expected frequency of febrile neutropenia within individual groups of diseases. It could be that certain diseases or chemotherapy regimen would benefit from either same day or next day administration of growth factor, and this would also be best determined in a prospective trial.
Despite these limitations, this is the first pediatric study to show that same day administration of Peg-GCSF after myelosuppressive chemotherapy does not cause increased rates of febrile neutropenia. This finding has the potential to decrease the length of hospitalizations, decrease the number of visits to outpatient clinics, decrease home health trips to patients’ homes, improve convenience for chemotherapy administration for families, and decrease healthcare costs. The increased use of a Peg-GCSF on-body injector Neulasta Onpro Kit has been frequently utilized in adult patients programmed to be administered more than 24 hours from the completion of chemotherapy. This could be a viable option for pediatric patients weighing more than 45 kilograms (kg). However, the syringes are prefilled at 6 milligrams (mg) which would be too much for a pediatric patient weighing less than 45 kg. There are no graduation marks on the syringes allowing for possible dosing errors, therefore administration is not recommended to patients who require dosing of less than 6 mg[5].
Conclusion
There was no statistically significant difference in the frequency of febrile neutropenia among pediatric patients when Peg-GCSF was given within 24 hours or greater than 24 hours from the completion of chemotherapy. Based on our findings, future prospective studies are needed in order to evaluate the efficacy and safety in administering Peg-GCSF within 24 hours following chemotherapy. This could potentially improve convenience for families and decrease health care costs.
Conflict of Interest: The authors declare that there is no conflict of interest.
Acknowledgments: We would also like to thank all of the providers across the United States and Canada for participating in our survey!
Abbreviations: Peg-GCSF – Pegfilgrastim; G-CSF - Granulocyte-Colony Stimulating Factors; FDA - Federal Drug Administration; EORTC - European Organisation for Research and Treatment of Cancer; GEE - General Estimating Equation; FDR - False Discovery Rate; Kg – Kilograms; Mg - Milligrams
References
- 1. Crawford, J., Ozer, H., Stoller, R., et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. (1991) N Engl J Med 325(3): 164-170.
- 2. Morstyn, G., Campbell, L., Souza, L.M., et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. (1988) Lancet 1(8587): 667-672.
- 3. Whitworth, J.M., Matthews, K.S., Shipman, K.A., et al. The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. (2009) Gynecol Oncol 112(3): 601-604.
- 4. Borinstein, S.C., Pollard, J., Winter, L., et al. Pegfilgrastim for prevention of chemotherapy-associated neutropenia in pediatric patients with solid tumors. (2009) Pediatr Blood Cancer 53(3): 375-378.
- 5. FDA. Center for Drug Evaluation and Research and Center for BIologics Evaluation and Research - Neulasta.
PubMed│CrossRef│Others
- 6. Burris, H.A., Belani, C.P., Kaufman, P.A., et al. Pegfilgrastim on the Same Day Versus Next Day of Chemotherapy in Patients With Breast Cancer, Non-Small-Cell Lung Cancer, Ovarian Cancer, and Non-Hodgkin’s Lymphoma: Results of Four Multicenter, Double-Blind, Randomized Phase II Studies. (2010) J Oncol Pract 6(3): 133-140.
- 7. Barni, S., Lorusso, V., Giordano, M., et al. A prospective observational study to evaluate G-CSF usage in patients with solid tumors receiving myelosuppressive chemotherapy in Italian clinical oncology practice. (2014) Med Oncol 31(1): 797.
- 8. Aapro, M.S., Bohlius, J., Cameron, D.A., et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. (2011) Eur J Cancer 47(1): 8-32.
- 9. Weycker, D., Li, X., Figueredo, J., et al. Risk of chemotherapy-induced febrile neutropenia in cancer patients receiving pegfilgrastim prophylaxis: does timing of administration matter? (2016) Support Care Cancer 24(5): 2309-2316.
- 10. Stokes, M.E., Davis, Charles, et al. Categorical data analysis using the SAS sytem. (2000) Cary, NC: SAS Institute, Inc.
PubMed│CrossRef│Others
- 11. Institute, S., Base SAS 9.4 procedures guids: Statistical procedures SAS Institute, 2016.
PubMed│CrossRef│Others
- 12. Benjamini, Y., Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. (1995) J Royal Stat Soc Series B (Methodological) 57(1): 289-300.
PubMed│CrossRef│Others
- 13. Abdi, H. Holm’s sequential Bonferroni procedure. (2010) Encyclopedia of Research Design 1(8): 1-8.
PubMed│CrossRef│Others