Perspectives of Raman imaging at sub-micrometer ranges
Affiliation
Department of Nanomaterials in Biomedicine, Regional Center of Advanced Technologies and Materials, Olomouc
Corresponding Author
Václav Ranc, Department of Nanomaterials in Biomedicine, Regional Center of Advanced Technologies and Materials, Olomouc, Czech Republic, Tel: +420 732 834 753; E-mail: vaclav@ranc.us
Citation
Václav, R. Perspectives of Raman Imaging at Sub-Micrometer Ranges (2016) Bioinfo Proteom Img Anal 2(2): 114-115.
Copy rights
© 2016 Václav, R. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Introduction
Optical microscopy has always played important roles in many research areas, including clinical research, food, agriculture, and history related research, and last, but not least, research related to environmental sciences. Essential advantages of optical imaging are recognized in its nondestructive mode of operation together with high achievable spatial resolution. Fluorescence imaging can be considered as a golden standard between imaging techniques, with its numerous advantages and few limiting disadvantages[1]. Two of the most important disadvantages are devised from the systemic introduction of fluorescent label, which commonly suffers from a photo bleaching and could (and frequently does) change physicochemical properties of the studied chemical system[2]. Raman spectroscopy is a powerful technique. Its main advantages, such as its ability for a simple combination with optical microscopy, nondestructive sample characterization, speed and information-rich obtained spectra are labeling this technique as a very promising alternative to a fluorescence imaging[3,4]. Raman spectra contain information about studied molecules in a form of a fingerprint, which in some cases indicates a current state of the molecule now of investigation. On the other hand, the ability of Raman imaging to primary work without labels could present a serious disadvantage, especially during the analysis of complex samples (biological, clinical), where Raman spectral data are overloaded with information about a state of many molecules. Consecutive extraction of a project-relevant data is than extremely challenging. Nonetheless, one of the most serious challenges, recognized in Raman spectroscopy, originates from its fundamental principle. Raman spectroscopy works on a principle of inelastic scattering of light with extremely small yields of photons possessing analytical information. Several techniques, including Coherent Anti-Stokes Raman Spectroscopy (CARS), Surface Enhanced Raman Spectroscopy (SERS), Tip Enhanced Raman Spectroscopy (TERS), have been developed to address this challenge.
CARS technique, discovered in 1965 by Maker and Terhune, presents an influence alternative to confocal Raman microscopy[5]. It belongs to a family of nonlinear technique, which means that multiple photons are involved in the scattering process at the same time. The scattering intensity is no longer linearly related to the intensity of the laser beam at high power densities. The main advantage of nonlinear techniques are its enhanced contrast and resolution along z-axis, used for depth profiling and thus its ability to construct 3D image maps[6]. However, serious disadvantages still exist and limit the use of CARS behind a research-scale level[7]. One of the limitations originates from the complex setup, necessary for the technique to suitably operate. The technique commonly uses two synchronized, mode-locked, solid state lasers focused on one single sample spot. Any misalignment leads to degrease of the analytical signal.
SERS was first described by Fleishmann in 1970[8]. It is based on the interaction of analyzed molecules with silver or gold nanoparticles / nanostructures. Small metal nanostructures with dimensions smaller than the wavelength of the incident light exhibit surface plasmons. The incident electromagnetic field can be strongly modified (in magnitude and orientation) in close vicinity to the metal nanostructure, especially if the incident wavelength is close to a plasmon resonance. This leads to the extraordinary enhancement of Raman intensity; usually enhancements of several orders of magnitude are observed. Tip enhanced Raman spectroscopy uses similar approaches[9]. The metal nanostructure is commonly positioned at the tip or at a tuning form of atomic force microscopy (AFM), hyphenated to Raman spectroscopy. The Raman laser beam is properly focused on the apex with a follow-up scan by the sample to keep the laser beam focused at the so called hot-spot. This technique has an extraordinary spatial resolution, commonly bellow 15 nm and increased sensitivity, given by the enhancement effects[10]. Larger-scale engagement of this technique outside research laboratories is still limited albeit many recognized advantages. Working setup utilizable for TERS is commercially available, however, challenges still exist in a reproducible and reliable preparation of TERS tips[11].
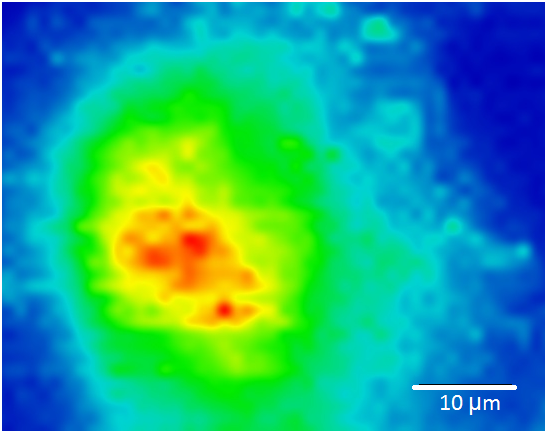
Raman imaging has already shown its great potential in many clinical, industrial or research applications, where cells, tissues, organs, nanostructures, novel materials, and others were studied. One of many examples is analysis of circulating tumor cells of breast cancer, performed in our research center. Previously isolated circulating tumor cells were analyzed using silver nanoparticles of diameter d = 28 nm, employed as SERS active substrate. Cells (n = 40 000) were deposited on a glass substrate and covered by a layer of Ag nanoparticles using a drop-coating method. Obtained spectral map with a spatial resolution 1 μm is displayed in the Figure 1. The color scheme represents a spectral intensity of Amide II band located at 1551 cm-1.
Figure 1: Raman image of circulating tumor cell. Color scheme represents abundance of spectral band, interpreted as Amide II (1551cm-1).
The selectivity and spatial resolution were greatly increased using modern approaches including CARS and TERS, however, there is still a great space for improvements. Modern trends and discoveries in the field of material chemistry and nano-manipulations can be helpful in a development of better TERS-tips and development in optics and electronics will help to produce less expensive lasers applicable in CARS.
References
- 1. Schäferling, M. The Art of Fluorescence Imaging with Chemical Sensors. (2012) Angew Chemie Int Ed 51(15): 3532–3554.
- 2. Rino, J., Braga, J., Henriques, R., et al. Frontiers in fluorescence microscopy. (2009) Int J Dev Biol 53(8-9-10): 1569–1579.
- 3. Smith, E., Dent, G. Modern Raman Sectroscopy - A Practical Approach. (2005).
- 4. Cheng, J-X., Xie, X.S. Vibrational spectroscopic imaging of living systems: An emerging platform for biology and medicine. (2015) Science 350(6264): aaa8870.
- 5. Maker, P.D., Terhune, R.W. Study of Optical Effects Due to an Induced Polarization Third Order in the Electric Field Strength. (1965) Phys Rev 137(3A): A801–A818.
- 6. Zhang, C., Zhang, D., Cheng, J-X. Coherent Raman Scattering Microscopy in Biology and Medicine. (2015) Annu Rev Biomed Eng 17(1): 415–445.
- 7. Tolles, W.M, Nibler, J.W., McDonald, J.R., et al. A Review of the Theory and Application of Coherent Anti-Stokes Raman Spectroscopy (CARS). (1977) Appl Spectrosc 31(4): 253–271.
- 8. Fleischmann, M., Hendra, P.J, McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. (1974) Chem Phys Lett 26(2): 163–166.
- 9. Domke, K.F., Pettinger, B. Studying Surface Chemistry beyond the Diffraction Limit: 10 Years of TERS.(2010) Chem Phys Chem 11(7): 1365–1373.
- 10. Deckert-Gaudig, T., Deckert, V. Tip-enhanced Raman scattering (TERS) and high-resolution bio nano-analysis—a comparison. (2010 ) Phys Chem Chem Phys 12(38): 12040.
- 11. Yang, Y., Li, Z-Y., Nogami, M., et al. The controlled fabrication of “Tip-On-Tip” TERS probes. (2014) RSC Adv 4(9): 4718–4722.