Phytochemical Analysis and Antioxidant Activity of the Flavonoids Extracts from Pods of Ceratonia siliqua L.
Ahmed Hariri
Affiliation
1Laboratory of Physical Chemistry of Macromolecules and Biological Interfaces, Faculty of Science the Nature and Life, University
of Mascara, Sidi Said, Mascara, Algeria
2Bioconversion Laboratory, Microbiology Engineering and Health Safety, Faculty of Science the Nature and Life, University of
Mascara, Sidi Said, Mascara, Algeria
Corresponding Author
Nawel Ouis, Laboratory of Physical Chemistry of Macromolecules and Biological Interfaces, Faculty of Science the Nature and Life, University of Mascara BP.763, Sidi Said, Mascara, Algeria; Tel: 00 213 6 61 72 11 90; E-mail: nawel_chim@yahoo.fr
Citation
Ouis, N., et al. Phytochemical analysis and antioxidant activity of the flavonoids extracts from pods of Ceratonia siliqua L. (2017) J Pharm Pharmaceutics 4(2): 159-165.
Copy rights
© 2017 Ouis, N. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Carob pod; Phytochemical analysis; Flavonoids; Antioxidant activity
Abstract
This study was carried out to determine the phytochemical profile and antioxidantactivity of the carob. The results of prelim inary phytochemical assessed showed that the alkaloid bases and salts, flavones aglycones, reducing compounds, fatty acids, polyterpenes, catechin tannins, volatile oils, cardiac glycosides, flavonoids, sterol glycosides and terpenes, amino acids and saponins are classes of chemical groups present in the pods of Ceratonia siliqua. The pods are characterized by 87 ± 1.0% dry matter, 55 ± 0.2 % total sugars, 4 ± 0.1 % proteins, 7 ± 0.4 % lipids, 4 ± 0.3 ash. We has found that the pod has a 10.56 ± 0.2 Na, 0.75 ± 0.1 Zn, 0.55 ± 0.3 Cu, 41.7 ± 0.2 Mg, 210 ± 0.3 Ca and 1150 ± 0.2 mg/100 g K. Pods of carob present 71.6 ± 2.10 mg/100 g of flavonoids, 56.51 ± 1.02 mg/100 g of alkaloids, 41.25 ± 1.36 mg/100 g of saponins and 1.18 ± 0.2 mg/100g of tannins. Flavonoids fractions were extracted using organic solvents with different polarity. The ethyl acetate extract has the highest content of polyphenols and flavonoids 259.4 ± 4.2, 71.34 ± 1.08 mg EGA/g E, followed by the n-butanol extract 62.19 ± 0.13, 53.01 ± 0.11, then the crude extract 15.5 ± 0.21, 12.9 ± 0.17 and finally aqueous extract 13.39 ± 0.27, 11.13 ± 0.62. The flavonoids fractions extracted by Ethyl Acetate and n-Butanol showed the higher antioxidant capacity determined by three methods: free radical scavenging activity, reducing power and liver lipid peroxidation compared to the crude exact and aqueous extract.
Introduction
Carob (Ceratonia siliqua L.), belongs to the subfamily Caesalpinaceae of the Leguminoseae family is a typical tree which has been widely grown in the Mediterranean region[1] and has an economic and environmental importance in Algeria. This specie is used in reforestation of arid and degraded areas and also as for ornamental purposes[2]. However, in recent years, it has been used in the food industry as bioresource and biomass substrate and thus has attracted the attention of producers because of increasing market value. It produces edible pods used as a fodder for breeding cattle; it has also a long history of application as a source of health products. The pulp and the seeds have some interesting properties and are often used in food and pharmacological industry[3]. The pulp content in the pod ranges from 73 to 95%[4]. The pod of the carob has a high energy value 17.5 kJ/g D.M[2,5] and has long been used as a feed for livestock and human consumption, including sweets, biscuits, and traditional carob concentrate called “pekmez”[6]. When the fruits are ripe enough, they have 91 – 92% total dry matter and 62 – 67% total soluble solids, which consist of 34 – 42% sucrose, 10 – 12% fructose, and 7 – 10% glucose. Carob pods are also characterized by high sugar content 500 g/kg[3]. Moreover, carob pods contain appreciable amount of fiber (4.2 - 39.8%), depending on the type of the extracted fiber[4]. The carob is rich in calcium, antioxidants and polysaccharides. Carob also contains phenolic compounds from 2 to 20 % D.M.[3,7] Carob pulp is a good source of polyphenols: mainly tannins 16 - 20%[2,7,8], and protein (2.7 - 7.6%) but it is poor in lipid (0.4 - 0.8%). The pulp and the seeds are valorized in different applications. The locust bean gum is also applied in pharmaceutical industry as drug delivery[9]. This study was aimed to determine the phytochemical analysis and antioxidant potential of the flavonoids extracts from the pods of Ceratonia siliqua.
Materials and Methods
Vegetable material and extraction
The pods of carob were collected in the month of June 2014 from the region of ELBORDJ (Mascara, Algeria). The species was authenticated by botanist at SNV faculty, University of Mascara. The collected pods (pulp and seeds) were washed with tap water to remove all impurities and then with distilled water. The samples were dried at room temperature and chopped into small particles to increase a surface of diffusion and the mass transport characteristics of the particular compounds. 20 g of dried carob pods were separately extracted with 150 mL of different solvents (chloroform, methanol and distilled water) for 72 hours under magnetic stirring. This soaking was repeated three-folds by renewing the solvent every 24 hours. Maceration of each solvent were combined and concentrated to 80 mL under reduced pressure using rotary evaporator. The samples to be tested were stored in refrigerator at 4°C.
Summary phytochemical analysis
The preliminary phytochemical components of Ceratonia siliqua were screened on the basis of staining characteristics tests to knowledge the major chemical groups. For this purpose, several types of reagents were used.
Quantitative phytochemical analysis
Primary metabolites: The total dry matter was determined by drying 1 g of sample to constant weight at 105°C using a vacuum drier[10]. The ash content was determined according to the AOAC official method 972.15 by incineration one g of dry sample for 3 hours at 600°C[10]. Mineral concentrations (Na, K, Mg, Ca, Cu and Zn) in mg/100 g of the carob pods were determined using an Atomic Absorption Spectrophotometer (Varian Spectra A-550 plus) and calculated using a standard curve[11]. Organic matter is the difference between the sample (dry matter) and ash resulting. The rate of lipids was determined using a Soxhlet-type apparatus. Total nitrogen and protein content were determined by the method of Kjeldahl digestion and distillation apparatus; a conversion factor of 6.25 was used to obtain the protein content[10]. Concentration of sugars was determined colorimetrically at 480 nm by Dubois method[12]. Standards were prepared with glucose solutions at different concentrations.
Secondary metabolites: Dried pods of carob were ground into a fine powder using a homogenizer. For different dosages of secondary metabolites, 2 g of powdered pods were defatted with 100 mL of diethyl ether for 2 hours using a soxhlet apparatus.
Test for flavonoids: Five g of the pods of carob were mixed with 50 mL of 80% methanol at room temperature. The mixture was then quickly filtered and then re-extracted for the second and the third time with the same solvent. The filtrates obtained were evaporated to dryness obtaining a residue[13].
Test for tannins: The extraction of the tannins was carried out by 70% acetone. 10 g of pods were soaked in 100 mL of solvent for 30 minutes. The operation was repeated three-folds. After filtration and evaporation, the dry residue was weighed to calculate the yield of tannins[14].
Test for saponins: Ten g of carob were dispersed in 100 mL of ethanol 20%. The extraction was carried out in a water bath at 55°C under agitation. This step lasts 4 hours was repeated a second time to the residue obtained after filtration. The collected filtrates were concentrated to volume equal to 40 mL. Then performs a series of liquid-liquid extraction, the first was carried by 20 mL of diethyl ether by repeating this operation. After the ethereal layer was removed and the second liquid-liquid extraction was begun by the n-butanol, adding 40 mL of the latter in the aqueous phase obtained after extraction with ether. This extraction was repeated three-folds again. The n-butanol phase was washed twice with 20 mL of 5% NaCl and then concentrated to a dry residue which expresses the weight yield saponins.
Test for alkaloids: Five g of the sample were weighed and 200 mL acetic acid in 10% ethanol was added, cover and let stand for 4 hours. Filter and concentrate the extract to a water bath at a quarter of the initial volume. Concentrated ammonium hydroxide was added drop wise to the extract until complete precipitation. The collected precipitate was washed with a dilute solution of ammonium hydroxide and then filtered. The residue is the alkaloid, which was dried and weighed[15].
Extraction of the flavonoids: The method as described by Merghem et al.[16] was employed for the extraction of flavonoids using organic solvents of increasing polarity. According to this method, 100 g of the carob pods powder were made up to 1 liter with methanol/distilled water (85/15 v/v). The mixture was subjected to stirring overnight at 4°C and then allowed to stand for several hours. The floating phase was subsequently filtered and stored at 4°C. The extraction was repeated once and the precipitate was added to 1 liter 50% methanol to yield the filtrate which was mixed with the first filtrate. The hydro alcoholic maceration were then combined and evaporated to dryness under vacuum using a rotary evaporator. The dry residue was taken up in boiling distilled water (200 mL) which quantitatively solubilizes phenolics compounds; decantation for 12 hours and followed by filtration to eliminate the “sludge” (fats, resin). Crude Extracts (CrE) thus obtained were subjected to several extractions with various organic solvents: Diethyl Ether (DE) removes chlorophyll pigments, carotenoids and fat and all non-phenolic compounds; Ethyl Acetate (EA) removes mono-o-glucoside and partially di-o-glucosides; Butanol (Bt) will cause the rest essentially of di-o-glycoside, tri-o-glycosides and c-glycosides. The aqueous phase (Aq) and the solvent were mixed thoroughly by leaving out every time the product gases. After standing for an hour and a half, the water phase and the solvent used in charge of its specific compounds were recovered separately. For each solvent, we again two or three time this operation for optimal training separate polyphenolic groups. After several washes, it also takes the remaining aqueous phase containing flavonoids.
The yield percentage of each extract was calculated as follows:
(Final weight of dried extract)
Yield % = -------------------------------------- * 100
(initial weight of carob powder)
Dosage of polyphenols and flavonoids
The method proposed by Miliauskas et al.[17] was employed for spectrophotometrically quantification of polyphenols concentration. The determination of flavonoids extracted was carried out by the colorimetric method as described by Ardestani and Yazdanparast[18]. Results are expressed in equivalent mg catechin per gram of dry vegetable matter (mg EC/g E).
Evaluation of antioxidant activity
No single method is appropriate to estimate the total antioxidant capacity of a sample, due to the variability of active compounds composition and the conditions of the test used. Antioxidant capacity methods can be divided into two groups depending on the following two chemical reactions: assays based on Hydrogen-Electron Transfer (HAT) and assays based on Single-Electron Transfer (ET). The antioxidant activity of the flavonoids extracts was evaluated by various antioxidant assays, including 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) radical-scavenging activity, reducing power and lipid peroxidation assay.
DPPH free radical-scavenging activity
The ability of the carob fractions to scavenge DPPH free radicals was assessed by the method described by Kirby and Schmidt[19] with some modifications. The substances to be tested for their antiradical power were prepared in methanol to achieve the concentration of 1 mg/mL. Dilutions were made to obtain concentrations of 500, 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90, 1.99 and 0.97 μg/mL. A volume of 50 μL of these dilutions was added to 1950 μL of methanol solution of DPPH (6.10-5 M) as free radical source. The mixtures were stirred vigorously for 30 seconds and then incubated for 30 min in the dark at room temperature. Scavenging capacity was recorded by monitoring the decrease in absorbance at 517 nm against a blank consisting of pure methanol. Ascorbic acid and catechin were used for comparison. Lower absorbance of the reaction mixture indicated higher free radical-scavenging activity. DPPH radical-scavenging activity was calculated as:
(A of control - A of sample)
% inhibition = ------------------------------------* 100
(A of control)
Control simple contained all the reagents except the carob extracts. Percentage of inhibition was calculated using the equation as described previously, whilst IC50 (concentration of substrate that inhibits 50% of the DPPH radicals present in the reaction medium) values were estimated from the % inhibition versus concentration plot, using a non-linear regression algorithm. Tests were carried out in triplicate.
Ferric-reducing activity
The reducing power of all extracts was determined by the method described by Yildirim et al.[20]. 1 mL of carob extract at different concentrations was mixed with 2.5 mL of 0.2 M phosphate buffer pH 6.6 and 2.5 mL of potassium ferricyanide solution K3Fe(CN)6, 1%. After incubation for 20 min at 50°C, 2.5 mL of trichloroacetic acid 10% was added and the reaction mixture was centrifuged for 10 min at 3000 rpm. An aliquot of 2.5 mL of the supernatant from each sample mixture was mixed in a test tube with 2.5 mL of distilled water and 0.5 mL of ferric chloride solution (0.1%) prepared freshly in distilled water. After 20 min of reaction time at 35°C, the absorbance was measured at 700 nm against a blank that contains all components except the extract solutions and ferric chloride. The control is achieved by different concentrations of ascorbic acid and catechin. Higher absorbance of the reaction mixture indicated higher reducing power. Tests were carried out in triplicate.
Liver Lipid peroxidation assay
Lipid peroxidation levels in the liver tissues were evaluated using the Thiobarbituric Acid Reactive Substances (TBARS) assay as described by the method of Tatiya and Saluja[21]. The mixture contained 0.5 mL of homogenate 10%, 1 mL of KCl (0.15 M) and 0.5 mL of various concentrations of each extract. The lipid peroxidation was initiated using 100 μL of ferric chloride 1 mM. After incubation for 30 min at 37°C, the reaction mixture was stopped by addition of 2 mL of iced HCl 0.25N containing 15% TAC Trichloroacetic Acid (tissue homogenate was deprotenized by TAC), 0.38% thiobarbituric acid, and 0.2 mL of Butylated Hydroxyl Toluene (BHT) 0.05%. The mixture was heated for 60 min at 80°C, cooled and centrifuged at 6900 rpm for 15 min. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (Cary 50, Varian, Palo Alto, CA, USA) against a blank containing all reagents with the exception of liver homogenate and extracts. Identical experiments were carried to determine the normal (without extract and FeCl3) and the level of lipid peroxidation in the tissues (with FeCl3 and without extract). For preparation of the homogenate, the liver was quickly removed after dissection of the rat’s, rinsed with physiological saline and homogenized at 4°C in a solution of KCl 0.15 M at 10%. The homogenate was centrifuged at 800 rpm for 15 min to remove cellular debris; the supernatant was recovered to examine the in vitro lipid anti peroxidation. The percentage effect Anti Lipid Peroxidation (% ALP) was calculated by the following formula:
(A of FeCl3 - A of sample)
% ALP = ------------------------------ * 100
(A of FeCl3 - A of normal)
Results
Preliminary phytochemical screening The results of preliminary phytochemical analysis, presence and absence in pods of Ceratonia siliqua was shown in table 1. The results revealed that the starch, polyuronides, anthocyanins, anthracenosids, emodols and carotenoids are chemical families completely absent in the pods of carob. It is clear from this analysis that the alkaloid bases and salts, polyterpenes, flavones aglycones, volatile oils, fatty acids, catechin tannins, cardiac glycosides, flavonoids, reducing compounds, sterol glycosides and terpenes, amino acids and saponins are classes of chemical groups present in the pods of Ceratonia siliqua.
Table 1: Phytochemical screening of chloroform, methanol and aqueous extracts of carob pods (+: presence, -: absence).
| Chloroform extract | Values | Methanol extract | Values | Aqueous extract | Values |
|---|---|---|---|---|---|
| Alkaloid bases | + | Alkaloid salts | + | Saponins | + |
| Sterols | + | Catechin tannins | + | Polyuronides | - |
| Polyterpenes | * | Gallic tannins | * | Starch | - |
| Carotenoids | - | Cardiac glycosides | + | ||
| Coumarins | + | Flavonoids | + | ||
| Flavones aglyones | + | Reducing compound | + | ||
| Emodols | - | Anthracenosides | - | ||
| Volatile oils | + | Anthocyanins | - | ||
| Fatty acids | + | Amino acids | + | ||
| Sterol | + | ||||
| Glycosides | + | ||||
| Terpenes | + |
Quantitative phytochemical analysis
Primary metabolites As shown in table 2, the percentage of the dry matter of the carob pods was estimated to the 87 ± 1.0%. Sugars are the abundant components in the carob pods; the content of this component was evaluated to the 55 ± 0.2%. The level of the protein and lipids was esteemed respectively to 4 ± 0.1% and 7 ± 0.4%. Ash is the total amount of minerals present in a sample, the value of the carob pods is in the order of 4 ± 0.3. This mineral salt was mainly constituted by Na (10.56 ± 0.2), Zn (0.75 ± 0.1), Cu (0.55 ± 0.3), Mg (41.7 ± 0.2), Ca (210 ± 0.3) and K (1150 ± 0.2) mg/100 g of the carob pods.
Table 2: Quantitative phytochemical characteristics of the carob.
| Primary metabolites | Values | Secondary metabolites | Values |
|---|---|---|---|
| Dry matter content % | 87 ± 1.0 | Flavonoids mg/100 g | 71.6 ± 2.10 |
| Organic matter % | 83 ± 0.6 | Alkaloids mg/100 g | 56.51 ± 1.02 |
| Total sugar content % | 55 ± 0.2 | Saponins mg/100 g | 41.25 ± 1.36 |
| Protein content % | 4 ± 0.1 | Tannins mg/100 g | 1.18 ± 0.2 |
| Lipid content % | 7 ± 0.4 | ||
| Titratable acidity % | 4 ± 0.2 | ||
| pH | 5.6 ± 0.1 | ||
| Ashes % | 4 ± 0.3 | ||
| Na (mg/100 g of the carob pods) | 10.56 ± 0.2 | ||
| Zn (mg/100 g of the carob pods) | 0.75 ± 0.1 | ||
| Cu (mg/100 g of the carob pods) | 0.55 ± 0.3 | ||
| Mg (mg/100 g of the carob pods) | 41.7 ± 0.2 | ||
| Ca (mg/100 g of the carob pods) | 210 ± 0.3 | ||
| K (mg/100 g of the carob pods) | 1150 ± 0.2 |
Secondary metabolites: Pods of carob used in current study present 71.6 ± 2.10 mg/100 g of flavonoids, 56.51 ± 1.02 mg/100 g of alkaloids, 41.25 ± 1.36 mg/100 g of saponins and 1.18 ± 0.2 mg/100 g of tannins (Table 2).
Yield of extraction: The calculation of yields relative to the dry weight of the carob showed that the Crude Extract (CrE) represents the highest yield (4.32 ± 0.43%), followed by the aqueous extract (3.22 ± 0.13%), then the butanol extract (1.96 ± 0.1%) and finally extracted with ethyl acetate (1.12 ± 0.3%).
Content of total phenols and flavonoids in dry extracts: From the obtained results, the Ethyl Acetate Extract (EAE) has the highest content of polyphenols and flavonoids respectively (259.4 ± 4.2 and 71.34 ± 1.08 mg EGA/g E), followed by the N-Butanol Extract (BtE) with values of 62.19 ± 0.13 and 53.01 ± 0.11 mg EGA/g E, then the Crude Extract (CrE) with 15.5 ± 0.21 and 12.9 ± 0.17 mg EGA/g E and finally Aqueous Extract (AqE) with values of 13.39 ± 0.27 and 11.13 ± 0.62 mg EGA/g E.
Antioxidant activity
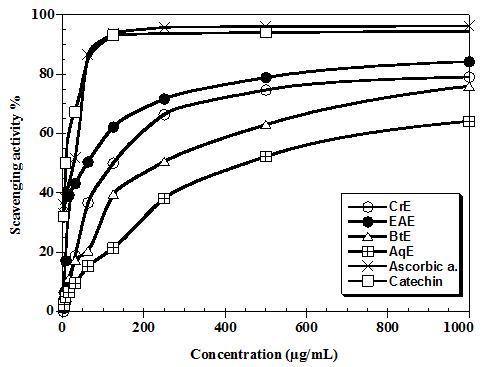
DPPH free radical-scavenging activity A shown in figure 1, at concentration of 1000 μg/mL, the Ethyl Acetate Extract (EAE) shows that it is most active with 84.3 ± 0.13% followed by the Crude Extract (CrE) 79.11 ± 0.9%, then the Butanol Extract (BtE) 76.01 ± 1.31% and finally the Aqueous Phase (AqE) 64.16 ± 1.32%. Ascorbic acid and catechin showed a high scavenging capacity of free DPPH radical respectively 96.23 ± 3.12% and 94.5 ± 2.78%. In term of IC50, the ethyl acetate extract present IC50 62.5 μg/mL, followed by crude extract 125 μg/mL, then butanol extract 250 μg/mL and aqueous extract 450 μg/mL. These values are highest compared to IC50 obtained by ascorbic acid and catechin respectively 30.21 μ6g/mL and 7.81 μg/mL.
Figure 1: Free radical-scavenging activity of the flavonoids extracts of Ceratonia siliqua measured by DPPH assay. Values presented are the means of triplicate analysis. (EAE: Ethyl acetate extracts, BtE: Butanol extracts, CrE: Crude extract, AqE: Aqueous extract).
Reducing power
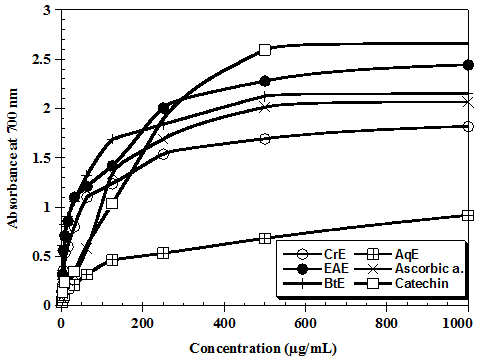
According to the figure 2, the reducing capacity of the flavonoids extracts of aqueous phase showed a very low activity for reducing iron, values observed by optical density not exceeding 0.9 at concentration 1000 μg/mL. The Ethyl acetate fraction provides an optical density of 2.44 ± 0.12 higher to OD obtained by ascorbic acid at the same concentration (1000 μg/mL). We can classify power reduction of iron by different fractions as follows: Catechin, ethyl acetate phase, butanol phase, ascorbic acid, crude extract, and aqueous extract.
Figure 2: Antioxidant capacities of Ceratonia siliqua using ferric reducing power method.
Liver Lipid peroxidation
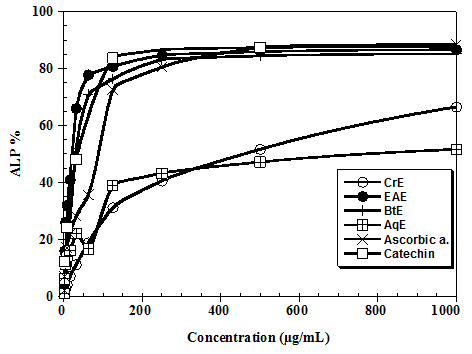
The inhibitory effect of the flavonoids extract of carob pods on Fe2+ induced lipid peroxidation in rat’s homogenates is shown in figure 3. The butanol and ethyl acetate extracts of Ceratonia siliqua protects against lipid peroxidation induced by Fe2+, considerably increase percentage of anti lipid peroxidation in a dose-dependent manner and are close to those of ascorbic acid and catechin with a percentage more than 80% at 1000 μg/mL. The aqueous and crude extract shows a low power anti lipid peroxidation.
Figure 3: Antioxidant capacities of the flavonoids extract of Ceratonia siliqua.
Discussion
The results of preliminary phytochemical analysis indicate the important value of the pods of carob studied. Ours results are close to those obtained by Torun et al.[22]. According to the study of the several authors, gallic acid is the most abundant phenolic acid in carob pods and their products[7,23,24]. One of the reasons for this high gallic acid content may be its release from tannins during the extraction process[25,26].
The content of the dry matter obtained is almost identical to those observed in several works[1,27,28]. The percentage of the total sugars obtained from pods of carob 55 ± 0.2% is lower than that specified by Biner et al.[2], 75% and it is higher than that carried out by Frentz and Zert[29] which is 40% value. Carob pods are known as poor in protein and lipids. Ours samples of carob are characterized by high protein content 4 ± 0.1% compared to value of 1 to 2% reported by Sbay and Abourouh[30] and lower to that carried out by Frentz, & Zert[29] and by Naghmouchi et al.[31]. According to ours results, the rate of the lipid was recorded to 7 ± 0.4%, this value is greater than that found by Owen et al[7], which is 4.80%. While the result is much higher than those obtained by Yousif and Alghzawi[1], which is 0.74% for carob of Jordan and 0.6% for carob of Italy. Ours results are similar to values obtained by Avallone et al[5], which is 6.6% for the most part represented by oleic acid (34.4%) and linoleic acid (44.5%), whereas palmitic acid (16.2%) and stearic acid (3.4%) were the major saturated fatty acids. The rate of ash obtained exceeds the range of 2% to 3% obtained by Dakia et al.[6], and against our value is less than 8.83%[32]. This can be explained by the geographical origin of the samples, including climate conditions and soil characteristics. The content of the mineral salts exceeds to those obtained by El Batal et al[33] 0.36 to 0.99. The magnesium content of the carob pods is 41.7 ± 0.2 mg/100 g MF. It is below the values 66.89 mg/100 g, 60 mg/100 g indicated by Eman et al[34]. The results of primary metabolites assay show that our carob pods have good potential nutrient.
Phenolic acids and flavonoids are secondary metabolites that are synthesised by plants during development, which possess an array of health-promoting benefits. The high level of flavonoids obtained in carob pods 71.6 ± 2.10 mg/100 g, reveal that this material is good managements of cardiovascular disease and oxidative stress. Ours results are higher to those obtained by El Kahkahi et al[27] and by Ayaz et al[35], the authors founds the most important flavonoids content of 41 to 48 mg/100 g of DM. The high content of alkaloids in carob pods 56.51 ± 1.02 mg/100 g can explain the traditional therapeutic uses reported by several authors. Low levels of tannins 1.18 ± 0.2 mg/100 g indicate non-toxicity of the carob. El Kahkahi et al[27], reports that the content of hydrolysable tannins in the pulp, seed Meknes (P3), pulp Khémisset (P4) and pulp Marrakech (P7) is the highest rate with 7 mg/100 g followed pulps Marrakech (P5) and pulp Fez (P2) with a rate of 6 mg/100 g pulp. Our results are similar than those of Saura Calixto[36] with 1.3% and lower than those of Avallone et al[5] with 95 mg/100g represented by ellagitannins and gallotannins. Phenolic compounds are known as powerful antioxidants. They are very important components in the extracts, and their ability of scavenging of free radicals is due to their hydroxyl groups. Ours results of polyphenols content are very high to theses obtained by Meziou-Chebouti et al.[28].
Flavonoids are phenolic compounds with well-known antioxidant activity. The antioxidant activity of each fraction was determined in terms of the percentage of DPPH scavenged, and in terms of the IC50, the concentration of extract required to decrease by 50% the initial DPPH concentration. The high antioxidant contents of vegetables and fruits have been linked to the inhibition of diseases associated with oxidative damage, such as coronary heart disease, stroke, and cancers. The present study showed that at concentration of 1000 μg/mL, the all flavonoids fractions of Ceratonia siliqua have an average antioxidant activity not exceed 84.3% compared to the ascorbic acid and catechin. Ours results are high to those obtained by Torun et al[22]. From the obtained results, the Ethyl Acetate Extract (EAE) has the highest content of polyphenols and flavonoids and showed the highest scavenging capacities of free radical DPPH and reducing power of iron compared to the others extract. Liver lipid peroxidation is associated with a loss of membrane fluidity and an increase of membrane permeability, causing a decrease in physiological performance. The butanol and ethyl acetate extracts of Ceratonia siliqua are effective in inhibiting the lipid peroxidation induced by the system Fe2+ ascorbate in rat’s homogenate. The generation of Malondialdehyde (MDA) and related substances which react with the thiobabiturique acid are inhibited by extracts[21]. This indicates significant activity of inhibiting lipid peroxidation of extracts. The preventative effects demonstrated by the extract could be due to the presence of antioxidant compounds.
Acknowledge:
We gratefully acknowledge Pr. I. Chevalot and F. Blanchard to have me to help to realize this work as well as all the team of the laboratory LRGP, ENSAIA, Nancy, France.
Conflict of interest:
The authors declare no conflicts of interest.
References
- 1. Yousif, A.K., Alghzawi, H.M. Processing and characterization of carob powder. (2000) Food Chem 69(3): 283–287.
Pubmed || Crossref || Others - 2. Biner, B., Gubbuk, H., Karhan, M., et al. Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. (2007) Food Chem 100(4): 1453–1455.
Pubmed || Crossref || Others - 3. Markis, D.P., Kefalas, P. Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. (2004) Food Technol Biotechnol 42(2): 105–108.
Pubmed || Crossref || Others - 4. Shawakfeh, K., Ereifej, K.I. Pod Characteristics of two Ceratonia siliqua L. Varieties from Jordan. (2005) Ital J Food Sci 17(2): 187–194.
Pubmed || Crossref || Others - 5. Avallone, R., Plessi, M., Baraldi, M. et al. Determination of chemical composition of carob (Ceratonia siliqua): protein, fat, carbohydrates, and tannins. (1997) J Food Composition Analysis 10: 166–172.
Pubmed || Crossref || Others - 6. Dakia, P., Wathelet, B., Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. (2007) Food Chem 102(4): 1368–1374.
Pubmed || Crossref || Others - 7. Owen, R.W., Haubner, R., Hull, WE. et al. Isolation and structure elucidation of the major individual polyphenols in carob fibre. (2003) Food Chem Toxicol 41(12): 1727–1738.
Pubmed || Crossref || Others - 8. Haddarah, A., Ismail, A., Bassal, A. et al. Morphological and chemical variability of Lebanese carob varieties. (2013) European Sci J 9(18): 353-369.
Pubmed || Crossref || Others - 9. Sandolo, C., Coviello, T., Matricardi, P. et al. Characterization of polysaccharide hydrogels for modified drug delivery. (2007) Eur Biophys J 36(7): 693–700.
Pubmed || Crossref || Others - 10. AOAC. Official methods of Analysis of AOAC international, Gaithersburg, Maryland, 2006.
Pubmed || Crossref || Others - 11. James, G.S. Analytical chemistry of foods. (1995) Lon¬don: Blackie Academic and Professional. 224 p.
Pubmed || Crossref || Others - 12. Dubois, M., Gilles, K.A., Hamilton J. K., et al. Colorimetric method for determination of sugars and related substances. (1956) Anal Chem 28(3): 350-356.
Pubmed || Crossref || Others - 13. Okwu, DE. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. (2005) Int J Mol Adv Sci 1(4): 375-381.
Pubmed || Crossref || Others - 14. Troszynsta, A., Ciska, E. Phenolic compounds of seeds coats of white and coloured varieties of Pea (Pisum sativum L.) and their total antioxidant activity. (2002) Csech J Food Sci 20: 15-22.
Pubmed || Crossref || Others - 15. Doherty, V.F., Olaniran, O.O., Kanife, U.C. Antimicrobial Activities of Aframomum Melegueta (Alligator Pepper). (2010) Int J Biol 2(2): 126-131.
Pubmed || Crossref || Others - 16. Merghem, R., Jay, M., Viricel, MR. et al. Five 8-C-benzylated flavonoids from Thymus hirtus (Labiatea). (1995) Phytochem 38: 637-640.
Pubmed || Crossref || Others - 17. Miliauskas, G., Venskutonis, P.R., Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extract. (2004) Food Chem 85: 231-237.
Pubmed || Crossref || Others - 18. Ardestani, A., Yazdanparast, R. Inhibitory effects of ethyl acetate extract of Teucrium polium on in vitro protein glycoxidation. (2007) Food Chem Toxicol 45: 2402-2411.
Pubmed || Crossref || Others - 19. Kirby, A.J., Schmidt, R.J. The antioxidant activity of chinese herbs for eczema and of placebo herbs. (1997) J Ethnopharmacol 56(2): 103–108.
Pubmed || Crossref || Others - 20. Yildirim, A., Mavi, A., Kara, A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. (2001) J Agric Food Chem 49(8): 4083–4089.
Pubmed || Crossref || Others - 21. Tatiya, A.U., Saluja, AK. Evaluation of phytochemical standards and in vitro antioxidant activity of tannins rich fraction of stem bark of Bridelia retusa. (2010) Int J PharmTech Res 2(1): 649-655.
Pubmed || Crossref || Others - 22. Torun, H., Ayaz, FA., Colak, N., et al. Phenolic acid content and free radical-scavenging activity of two differently processed carob tree (Ceratonia siliqua L.) pod. (2013) Food Nutr Sci 4: 547-553.
Pubmed || Crossref || Others - 23. Ayaz, F.A., Torun, H., Ayaz, S. et al. Determination of chemical composition of Anotolian carob pod (Ceratonia siliqua L.): sugars, amino and organic acid, minerals and phenolic Compounds. (2007) J Food Quality 30(6): 1040-1055.
Pubmed || Crossref || Others - 24. Papagiannopoulos, M., Wollseifen, HR., Mellenthin, A., et al. Identification and quantification of polyphenols in carob fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MSn. (2004) J Agri Food Chem 52(12): 3784-3791.
Pubmed || Crossref || Others - 25. Ortega, N., Macia, A., Romero, MP. et al. Rapid determination of phenolic compounds and alkoloids of carob flour by improved liquid chromatography tandem mass spectrometry. (2009) J Agri Food Chem 57(16): 7239-7244.
Pubmed || Crossref || Others - 26. Rakib, E.M., Chicha, H., Abouricha, S. et al. Determination of phenolic composition of carob pods grown in different regions of Morocco. (2010) J Nat Pro 3(17): 134-140.
Pubmed || Crossref || Others - 27. El Kahkahi, R., Zouhair, R., Diouri, M. et al. Morphological and biochemical characterization of Morocco carob tree (Ceratonia siliqua L.). (2015) Int J Biol Med Res 6(2): 4946-4952.
Pubmed || Crossref || Others - 28. Meziou-Chebouti, N., Merabet, A., Behidj, N. et al. Chemical composition and antibacterial activity of Ceratonia siliqua L. growing in boumerdes (Algeria). (2015) Proc of the Int Conf on Biol and Biomed Eng (BBE 2015) Vienna, Austria 2(1): 96-99.
Pubmed || Crossref || Others - 29. Frentz, J.C., Zert, P. Territoires et alimentation. L’encyclopédie de la charcuterie. Ed. Soussana, (1990): 845.
Pubmed || Crossref || Others - 30. Sbay, H., Abourouh, M. Apport des espèces à usages multiples pour le développement durable : Cas du pin pignon et du caroubier. (2006) Centre de Recherche Forestière. Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification, Rabat (10): 1–9.
Pubmed || Crossref || Others - 31. Naghmouchi, S., Khouja, M.L., Khaldi, A. et al. Biochemical diversity of wild carob tree populations and its economic value. (2012) Topics Conserv Biol (25): 953–978.
Pubmed || Crossref || Others - 32. Puhan, Z., Wielinga, M.W. Products derived from carob pods with particular emphasis on carob bean gum (CBG). (1996) Report Technical Committee of INEC (unpublished) 12: 123-127.
Pubmed || Crossref || Others - 33. El Batal, H., Hasib, A., Ouatmane, A. et al. Yield and composition of carob bean gum produced from different Moroccan populations of carob (Ceratonia siliqua L.). (2013) J Mater Environ Sci 4 (2): 309-314.
Pubmed || Crossref || Others - 34. Eman, M., Salem D., Awlya Ohaad Fahad, A. Substituting of cacao by carob pod powder in milk chocolate manufacturing. (2012) Aus J Basic App Sci 6(3): 572–578.
Pubmed || Crossref || Others - 35. Ayaz, F.A., Torun, R.H., Glew, Z.D. et al. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. (2009) Plant Foods Hum Nutr 64(4): 286 292.
Pubmed || Crossref || Others - 36. Saura C.F. Effect of condensed tannins in the analysis of dietary fiber in carob pods. (1988) J Food Sci 53(6): 1769-1771.
Pubmed || Crossref || Others