Phytochemical screening and evaluation of antioxidant and cytotoxic activities of Halimeda opuntia.
Tamim Ahsan, Toufiqul Islam, Md. Abdul Alim, Md. Farzanoor Rahman, Mohammad Nazir Hossain*, Md. Morshedul Alam*
Affiliation
Department of Genetic Engineering and Biotechnology, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka-1216, Bangladesh.
Corresponding Author
Morshedul, M.A., Department of Genetic Engineering and Biotechnology, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka-1216, Bangladesh; E-mail: morshedbt@gmail.com
Citation
Morshedul, M.A., et al. Phytochemical screening and evaluation of antioxidant and cytotoxic activities of Halimeda opuntia. (2020) J Marine Biol Aquacult 6(1):1-6.
Copy rights
© 2020 Morshedul, M.A. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Antioxidant; Cytotoxic; Steroids;
Abstract
Bioactive properties of seaweeds isolated from the Bay of Bengal of Bangladesh have hardly been studied. In the present study, Halimeda opuntia, a calcareous seaweed, which was collected from the St. Martin’s Island, was used to investigate its antioxidant and cytotoxicity profile along with the phytochemical constituents by using the ethanolic and methanolic extracts. Through phytochemical screening, we confirmed the presence of phenolic compounds and steroids in all extracts. Soaking samples in solvent with occasional shaking for 5-7 days may lead to extraction of higher quantities of compounds and thus higher bioactivities compared with 2-2.5 hours of shaking. Using the well-known 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assay, we confirmed concentration-dependent DPPH scavenging activity. For cytotoxicity study, we used brine shrimp lethality assay and found considerable cytotoxicity highlighted by two ethanolic extracts that were highly toxic (LC50< 100 µg/mL) to brine shrimp Nauplii. So, H. opuntia can be a good source of novel and potent cytotoxic compounds possibly with anti-tumor properties, which should be further clarified. Thus, our study clearly suggested that due to the presence of considerable amounts of bioactive compounds, H. opuntia would be a valuable source of antioxidants with cytotoxic properties.
Introduction
Free radicals are one of the tycoons in the pathology of numerous diseases that are akin to various types of aging associated disorders due to lipid peroxidation, protein peroxidation, DNA damage and cellular degeneration in the cells[1]. Antioxidants scavenge these free radicals leading to the primary protection from chronic diseases. Though synthetic antioxidants are being used but the key concern is their health risks and acute toxicity. In this regard, natural antioxidant sources with least side effects are paramount and marine resources have received a great attention[2].
Halimeda is a genus of warm temperate to tropical calcareous macroalgae[3]. Various pharmacological properties like antibacterial[4-6], antifungal[5,7], hepatoprotective[8], cytotoxic[5] of this genus were previously reported, which were isolated from different parts of the ocean with biodiversity[4–9]. There is no report on the bioactive properties of Halimeda opuntia species from the Bay of Bengal of Bangladesh origin, a highly diversified continental shelf. There are at least 193 seaweed species in Bangladesh and a minimum of 140 of them are found in the St. Martin’s Island[10].
The aim of this study was to evaluate the antioxidant and cytotoxic assays to decipher the pharmacological effects and inspect phytochemicals of H. opuntia from the Bay of Bengal of Bangladesh. Though numerous studies have shown the pharmacological importance of this species, which have been done in different continental shelf of the world, there still remains ample scope for further research due to biodiversity. So far, for the first time an attempt was taken to abet the antioxidant and cytotoxic effect of its ethanolic and methanolic extracts in South Asian territory. Accordingly, we disclose herein the potent antioxidant and cytotoxic properties of H. opuntia to further establish the scientific basis of this species from the Bay of Bengal.
Results
Phytochemical Screening
All extracts contained phenolic compounds and steroids. EtOH (Preserve) and EtOH (Crude) had the highest quantity of both these compounds. Soaking the powdered samples in solvent with occasional shaking for 5-7 days extracted higher quantities of phenolic compounds and steroids than shaking them for 2-2.5 hours. Saponins were only detected in EtOH (Preserve) Table 1.
Table 1: Qualitative analysis of the phytochemicals of H. opuntia extracts.
|
Tested compounds |
|
EtOH |
MeOH |
|||
|
Preserve |
Crude |
Soak |
Shake |
Soak |
Shake |
|
|
Phenolic compounds |
+++ |
+++ |
++ |
+ |
++ |
+ |
|
Tannins |
- |
- |
- |
- |
- |
- |
|
Steroids |
+++ |
+++ |
++ |
+ |
++ |
+ |
|
Glycosides |
- |
- |
- |
- |
- |
- |
|
Flavonoids |
- |
- |
- |
- |
- |
- |
|
Alkaloids |
- |
- |
- |
- |
- |
- |
|
Saponins |
+ |
- |
- |
- |
- |
- |
(-): not detectable, (+): low quantities, (++): moderate quantities, (+++): high quantities
DPPH Scavenging Activity
Highest DPPH scavenging activity (21.04 ± 1.04 % of inhibition) was observed for EtOH (Preserve) at 3.34 mg/mL Table 2. None of the extracts caused 50% of inhibition. So, IC10 or IC15 was determined. We used both linear regression and best-fitting models (4- and 5-parameter logistic regression for EtOH (Preserve) and EtOH (Crude), respectively) for determining IC15. Values obtained from linear regression remained within the 95% confidence interval for IC15 estimated by 4- or 5-parameter logistic regression in both cases. Only linear regression was used to determine IC10 of other four extracts since fewer data points were available for them Table 3. Only 5-parameter logistic regression was used to determine IC10 and IC15 of L-ascorbic acid since its dose-response curve was hyperbolic (Figure 1A).
Table 2: DPPH scavenging activity of EtOH (Preserve), EtOH (Crude) and L-ascorbic acid
|
Sample |
Concentration (mg/mL) |
% inhibition |
IC15 (mg/mL)a |
IC15 (mg/mL)b |
AEACc (mg ascorbic acid equivalent/100 g) |
|
EtOH (Preserve) |
0.1255 0.2505 0.501 0.8905 1.336 1.781 2.672 3.34 |
4.21 ± 0.8 5.31 ± 0.55 5.76 ± 0.63 6.29 ± 0.44 8.48 ± 0.41 13.01 ± 1.59 15.14 ± 1.68 21.04 ± 1.04 |
2.4 ± 0.21 |
2.43 (2.09-2.77) |
32.72 ± 2.87 |
|
EtOH (Crude) |
0.1255 0.2505 0.501 0.8905 1.336 1.781 2.672 3.34 |
4.22 ± 0.15 3.54 ± 0.53 4.99 ± 0.17 4.38 ± 0.69 7.58 ± 0.48 10.59 ± 0.59 14.17 ± 0.67 19.94 ± 0.43 |
2.65 ± 0.11 |
2.68 (2.46-2.89) |
29.41 ± 1.27 |
|
L-Ascorbic Acid |
0.15 x 10-3 1.5 x 10-3 15 x 10-3 15.625 x 10-3 25 x 10-3 31.25 x 10-3 62.5 x 10-3 |
3.69 28.35 92.09 95.17 95.52 96.56 96.11 |
|
7.8 x 10-4 (6.98 x 10-4 -8.64 x 10-4) |
|
a calculated with linear regression. Values are expressed as mean ± SD (n=3)
b estimated with 4-parameter and 5-parameter logistic regression for EtOH (Preserve) and EtOH (Crude), respectively. 95% CIs are in the parantheses
c calculated using values derived from linear regression. Results are expressed as mean ± SD (n = 3)
Table 3: DPPH scavenging activity of EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake).
|
Extracts |
Concentration (mg dry weight/mL) |
% inhibition |
IC10 (mg dry weight/mL)* |
AEAC (mg ascorbic acid equivalent/100 g dry weight)* |
|
EtOH (Soak) |
12.5 25 50 |
1.38 ± 0.47 4.74 ± 0.68 15.83 ± 0.78 |
36.02 ± 1.7a |
1.42 ± 0.07a |
|
EtOH (Shake) |
12.5 25 50 |
1.67 ± 0.28 6.73 ± 0.45 12.18 ± 0.35 |
40.75 ± 1.25ab |
1.25 ± 0.04b |
|
MeOH (Soak) |
12.5 25 50 |
2.32 ± 0.41 7.1 ± 0.41 10.74 ± 0.49 |
44.58 ± 2.08bc |
1.14 ± 0.05c |
|
MeOH (Shake) |
12.5 25 50 |
1.95 ± 0.65 6.59 ± 0.51 9.96 ± 0.64 |
48.12 ± 2.9c |
1.06 ± 0.07c |
Values are expressed as mean ± SD (n=3).
*Values in the same column followed by different letter superscripts (a-c) are significantly different from each other (p <0.05). Differences between values in the same row followed by the same letter superscript are not statistically significant. p values have been adjusted for multiple comparisons
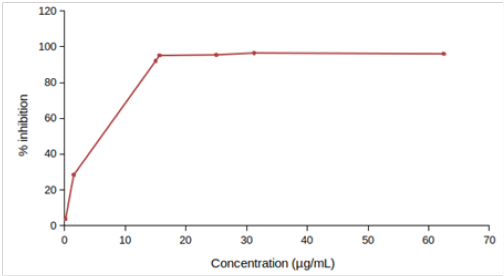
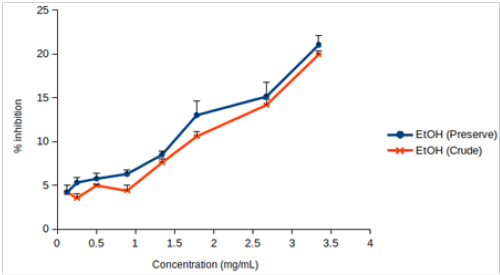
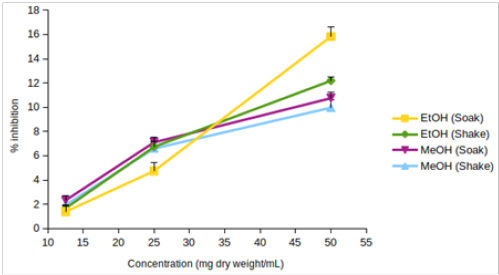
Figure 1: DPPH scavenging activity of (A) EtOH (Preserve) and EtOH (Crude), (B) EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake), (C) L-ascorbic acid. Values are represented as mean (error bars indicate standard deviation) (n=3).
EtOH (Crude) seemed to have lower DPPH scavenging activity than EtOH (Preserve) (Figure 1B). But IC15 and AEAC of these two extracts were not significantly different from each other (p >0.05). Among the other four extracts, EtOH (Soak) showed different pattern of inhibition (Figure 1C), significantly lower (p < 0.01) IC10 than both methanolic extracts (Figure 2A) and significantly higher (p < 0.05) AEAC (Figure 2B) than the other three extracts. Ethanolic extracts and extracts prepared by soaking method had significantly higher (p <0.05) DPPH scavenging activity than methanolic extracts and extracts prepared by shaking method, respectively, but solvents caused more differences in this activity than methods applied (Supplementary figure 1). Lines in the interaction plots for IC10 (Supplementary figure 2A) and AEAC (Supplementary figure 2B) of EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake) were not completely parallel to each other. But interaction between solvent and extraction method was not statistically significant (p > 0.05) (data not shown). So, extraction methods did not affect extraction by either solvent in a significantly different way.
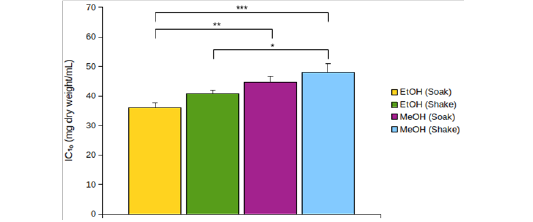
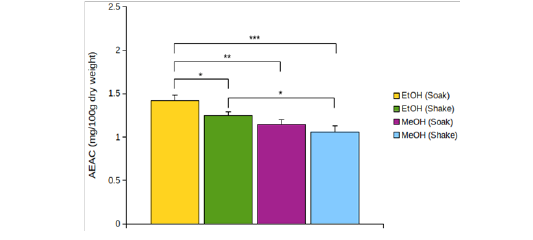
Figure 2: (A) IC10 and (B) AEAC of EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake). *p < 0.05, **p < 0.01, ***p < 0.001. p values were adjusted for multiple comparisons.
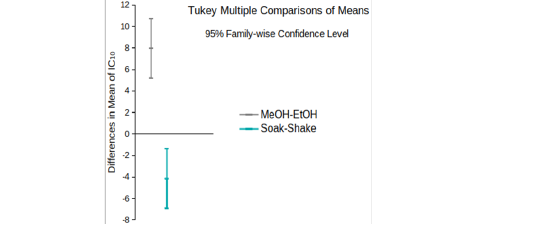
Supplementary figure 1: 95% confidence intervals of differences in the mean of IC10 of extracts prepared with ethanol and methanol and by soaking and shaking.
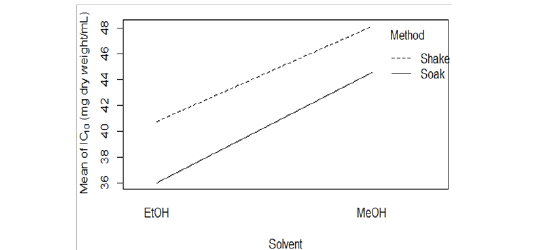
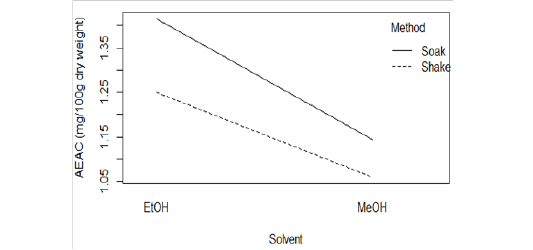
Supplementary figure 2: Interaction plot for (A) IC10 and (B) AEAC of EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake).
Brine Shrimp Lethality Assay
100% mortality of nauplii was caused by EtOH (Preserve), EtOH (Crude) and potassium dichromate (Table 4), but not by the other four extracts (data not shown). LC50 was estimated at 95% CI using LW1949 package[11] and also calculated by Probit regression analysis. Values calculated using the latter method always remained within the 95% CI estimated using the former method (Table 5).
Table 4: Mortality (%) of brine shrimp nauplii at various concentrations of EtOH (Preserve), EtOH (Crude), potassium dichromate and negative controls.
|
Extracts |
Concentration (µg/mL) |
Mortality (%) |
|
EtOH (Preserve) |
668 |
100 |
|
334 |
93.33 |
|
|
167 |
80 |
|
|
83.5 |
56.67 |
|
|
41.75 |
16.67 |
|
|
20.91 |
0 |
|
|
668 |
100 |
|
|
334 |
90 |
|
|
167 |
73.33 |
|
|
EtOH (Crude) |
83.5 |
53.33 |
|
41.75 |
16.67 |
|
|
20.91 |
0 |
|
|
90 |
100 |
|
|
81 |
100 |
|
|
72 |
93.33 |
|
|
63 |
86.67 |
|
|
Potassium Dichromate |
54 |
70 |
|
45 |
63.33 |
|
|
36 |
46.67 |
|
|
27 |
30 |
|
|
18 |
23.33 |
|
|
9 |
6.67 |
|
|
No mortality was observed in the Negative controls |
||
Table 5: LC50 and toxicity profiles of EtOH (Preserve), EtOH (Crude) and potassium dichromate
|
Extracts |
LC50 (µg/mL)a |
95% CI for LC50(µg/mL)a |
LC50 (µg/mL) (Probit Analysis)b |
Toxicity Profile |
|
|
|
|
|
|
Clarkson’s Toxicity Index |
Meyer’s Toxicity Index |
|
EtOH (Preserve) |
86.41 |
68.21-109.46 |
84.81 |
Medium/ Highly Toxic |
Toxic |
|
EtOH (Crude) |
94.24 |
72.6-122.34 |
92.63 |
Medium/ Highly Toxic |
Toxic |
|
Potassium Dichromate |
32.19 |
27.73-37.36 |
31.95 |
Highly Toxic |
Toxic |
a Estimated using Litchfield-Wilcoxon method with LW1949 package
b Calculated using probit analysis. Regression equations were generated with Libre Office Calc.
According to Clarkson’s toxicity index[12], extracts/compounds with LC50 value of 100-500 µg/mL are medium toxic and those with LC50 value of 0-100 µg/mL are highly toxic. So, potassium dichromatewas highly toxic. EtOH (Preserve) and EtOH (Crude) were medium to highly toxic. According to Meyer’s toxicity index[13], extracts with LC50< 1000 µg/mL are toxic. So, both of those extracts were toxic based on this criterion. Both extracts were significantly less toxic than potassium dichromate (Figure 3A). Yields were not determined for the other four extracts. So, toxicity profile could not be ascertained. 95% CIs for their LC50 overlapped with one another (Figure 3B). So, it cannot be deduced with certainty that their toxicities were significantly different from each other. Among those four extracts, 95% CIs for LC50 of ethanolic extracts were, in general, wider than those of methanolic extracts. So, the tested concentrations provided more precise information about the cytotoxicity of methanolic extracts.
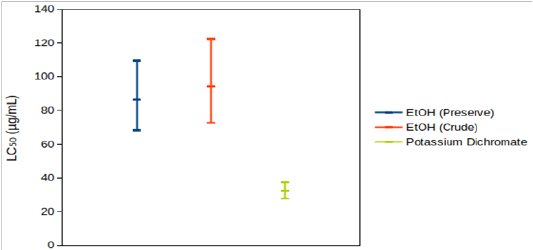
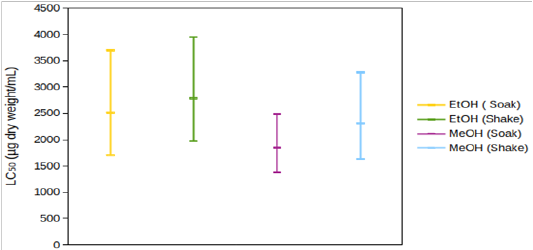
Figure 3: 95% confidence intervals for LC50 of (A) EtOH (Preserve), EtOH (Crude) and potassium dichromate, (B) EtOH (Soak), EtOH (Shake), MeOH (Soak) and MeOH (Shake).
Discussion
In this study we confirmed the presence of phenolic compounds,which is similar with the previous report[14], along with steroids in Halimeda opuntia,.We further clarified the antioxidant and cytotoxic properties of this species.
We found significantly higher DPPH scavenging activity by soaking method compared to shaking method in EtOH (Soak) and MeOH (Soak) (Table 2; Figure 1B, 1C, 2A and 2B). H. opuntia was exposed to sunlight and so was expected to have efficient antioxidant mechanisms due to the of phenolic compounds[14,15]. But 50% DPPH scavenging activity may not be obtained by its crude solvent extracts even at high concentrations[8]. It doesn’t necessarily indicate that H. opuntia doesn’t produce effective antioxidants. In fact, fractions of the extracts enriched in bioactive compounds may have better DPPH scavenging activity[16].
In brine shrimp lethality assay, all extracts showed cytotoxicity and two extracts- EtOH (Preserve) and EtOH (Crude)- were medium to highly toxic (Table 4 & 5 ), which is similar with a previous study[5]. Presence of phenolic compounds and steroids in all extracts may account for their toxicity to brine shrimps[17,18]. Generally, cytotoxic compounds in seaweeds, including H. opuntia , can act as chemical defense against herbivores[19,20]. Further studies are needed to develop a deeper understanding of cytotoxic compounds produced by H. opuntia and their anti-tumor properties.
In our phytochemical screening study, the highest quantity of phenolic compounds ensured the possible presence of antioxidant, anti-inflammatory, anti-allergenic, antithrombotic, anti-carcinogenic and hepatoprotective activities of H. opuntia . In addition, for the first time we disclosed the presence of steroids in this species, which will open a new avenue to explore the cellular signaling system by using H. opuntia as a source of steroids.
Materials and Methods
Sample Collection and Processing
Seaweed samples were collected in November, 2019 from the shallow water on the eastern side of Chera Island (CheraDwip), which is an indwelled extension of St. Martin’s Island. They were then cleaned with clean seawater and completely immersed in 50% ethanol for preservation. The collected seaweed was subsequently identified to be Halimeda opuntia (Linnaeus) J.V. Lamouroux based on its morphology[21]. After about one month, the seaweeds were taken out of ethanol, washed with filtered water and segmented followed by air drying and oven drying at 37º C, then ground to powder with mortar and pestle. The powder was stored at -20ºC until further use.
Preparation of Extracts
5.0 g of powdered sample was soaked in 50 mL of 50% ethanol and 70% methanol, separately, with occasional shaking for 5-7 days. Extracts prepared in this way were referred to as EtOH(Soak) and MeOH (Soak), respectively. 5.0 g of powdered sample was also separately shaken in 50 mL of 50% ethanol and 70% methanol at 25ºC and 150 rpm with a table top shaking incubator (Model : JSSI-070C, JSR, Korea) for 2-2.5 hours. Extracts thus prepared were referred to as EtOH (Shake) and MeOH (Shake), respectively. In all cases, after the stipulated time the resulting extracts were filtered through Double Rings 11.0 cm filter paper (Qualitative, 102). The initial concentrations of these four extracts were expressed as 100 mg dry weight/mL[22]. 60 mL of the 50% ethanol used for preservation was taken and the solvent was evaporated in oven at 45ºC to give amorphous solid masses[23], which were weighed and dissolved again in 60 mL of 50% ethanol and this extract was referred to as EtOH (Crude). The 50% ethanol for preservation was indicated as EtOH (Preserve). Both these extracts had a concentration of 6.68 mg/mL. Comparison between the composition and the bioactivities of these two extracts may give useful information about the thermostability of bioactive compounds of H. opuntia . All extracts were stored at -20º C until further use.
Phytochemical Screening
Qualitative phytochemical tests for the identification of phenolic compounds, tannins, alkaloids, steroids, steroidal glycosides, flavonoids and saponins were performed using previously described methods[24-27]. Phytochemical screening of the extracts was performed with following tests: phenolic compounds with lead acetate test, tannins with ferric chloride test, and alkaloids with Mayer’s test, steroids and glycosides with Salkowski’s test, flavonoids with alkaline reagent test and saponins with frothing test.
DPPH Scavenging Activity
DPPH scavenging activity was assayed using a previously described method[28,29] with slight modifications. In brief, 4 mg of DPPH (Cat. No.: sc-202591, Santa Cruz Biotechnology, USA) was dissolved in 100 mL of 95% methanol. 2 mL of the each extract, after dilution with respective solvent, was mixed with 2 mL of DPPH solution making the final concentrations for EtOH (Preserve) and EtOH (Crude) 3.34, 2.672, 1.781, 1.336, 0.8905, 0.501, 0.2505 and 0.1255 mg/mL and those of the other four extracts 50, 25 and 12.5 mg dry weight/mL. These mixtures were kept in the dark at room temperature for 1 hour. Then absorbance was measured at 517 nm using a UV-VIS spectrophotometer (model: UV-1900, Shimadzu). All determinations were performed in triplicates. The free radical scavenging activity or % of inhibition was calculated using the following formula:
% inhibition = (1- (Asample-Ablank)/(Acontrol-Ablank ) ) x 100
Where Asample= Absorbance of reaction in presence of the sample (sample dilution + DPPH solution)
Acontrol= Absorbance of control reaction (sample solvent + DPPH solution)
Ablank= Absorbance of blank for each sample dilution (sample dilution + DPPH solvent)
L-ascorbic acid was used as a positive control and DPPH scavenging activity of the extracts was also expressed as ascorbic acid equivalent antioxidant capacity (AEAC) (mg ascorbic acid/100 g dry weight).
Brine Shrimp Lethality Assay (BSLA)
Brine shrimp lethality assay was performed using previously described methods[30-32]with modifications. In brief, artificial seawater was made by dissolving 37 g sodium chloride 1 L of sterile distilled water and adjusting its pH between 8.25 and 8.5 by adding 1.0 N sodium hydroxide (NaOH). 200 mg of brine shrimp (Artemiasalina) eggs were hatched to produce nauplii in 1 L of this water for 24 hours under strong aeration in a vessel illuminated by a 60 watts bulb. 500 µL, 250 µL, 125 µL, 62.5 µL, 31.25 µL and 15.65 µL of each extract were transferred in triplicates into separate test tubes and organic solvent was completely evaporated. 10 nauplii were added to each test tube and the final volume was adjusted to 5.0 mL by adding artificial seawater. After 24 hours, number of dead nauplii was counted with the aid of a magnifying glass. Mortality (%) was calculated using the following formula
Mortality (%) = (number of dead nauplii)/(number of dead nauplii+number of alive nauplii) x 100
Cytotoxicity of the positive control, potassium dichromate (K2Cr2O7), was determined in the same way using final concentrations of 90, 81, 72, 63, 54, 45, 36, 27, 18 and 9 µg/mL in triplicates. 50% ethanol and 70% methanol were used as negative controls.
Statistical Analysis
Statistical analyses of the results were performed by using ANOVA (one & two-way), Tukey’s Honest Significant Difference (HSD) test and two-sample t-test. LC50 values were estimated at 95% confidence interval (CI) using Litchfield-Wilcoxon method[33] with LW1949 package of R. LC50 was also calculated using Probit Analysis. All the triplicate data were expressed as mean±SD as appropriate. The limit of significance was set at p<0.05.
Acknowledgement
This work was supported by the University Grants Commission (UGC) research grant, Bangabandhu Sheikh Mujibur Rahman Maritime University (BSMRMU), Dhaka, Bangladesh (2019-2020).
Conflict of interest
We have no conflicts of interest to disclose in this study.
References
- 1. Halliwell, B., Gutteridge, J.M., Cross, C.E. Free radicals, antioxidants, and human disease: where are we now? (1992) J Lab Clin Med 119(6):598–620.
- 2. Ma, C., Yang, K., Wang, Y., et al. Anti-aging Effect of Agar Oligosaccharide on Male Drosophilamelanogaster and its Preliminary Mechanism. (2019) Mar Drugs 17(11): 632.
- 3. Drew, E. Halimeda. (2011) 535–539.
- 4. Al-Judaibi, A. Antibacterial Effects of Extracts of Two Types of Red Sea Algae. (2014) J Biosci Med 02(02):74–82.
- 5. Selim, S.A. Antimicrobial , Antiplasmid and Cytotoxicity Potentials of Marine Algae Halimeda opuntia and Sarconema filiforme collected from Red Sea Coast. (2012) Int J Medical, Heal Biomed Bioeng Pharm Eng 6(1):79–84.
- 6. Mishra, J.K., Srinivas, T., Sawhney, S. Antibacterial Activity Of Seaweed Halimeda opuntia From The Coasts of Souths Andaman. (2016) Glob J Bio-Science Biotechnol 5(3):345–348.
PubMed│CrossRef│Others
- 7. Mtolera, M.S., Semesi, A.K. Antimicrobial activity of extracts from six green algae from Tanzania. (1996) Curr Trends Mar Bot Res East African Reg 211–217.
PubMed│CrossRef│Others
- 8. de Oliveira e Silva, A.M., Vidal-Novoa, A., Batista-González, A.E., et al. In vivo and in vitro antioxidant activity and hepatoprotective properties of polyphenols from Halimeda opuntia (Linnaeus) Lamouroux. (2012) Redox Rep 17(2):47–53.
- 9. Hendri M. Antibacterial Potential Screening of Halimeda sp on Some Types of Pathogenic Bacteria. (2015) Int J Mar Sci 5(53):1–6.
PubMed│CrossRef│Others
- 10. Sarkar, M.S.I., Kamal, M., Hasan, M.M., et al. Present status of naturally occurring seaweed flora and their utilization in Bangladesh. (2016) Res Agric Livest Fish 3(1):203–216.
- 11. Adams, J.V., Slaght, K.S., Boogaard, M.A. An automated approach to Litchfield and Wilcoxon’s evaluation of dose-effect experiments using the R package LW1949. (2016) Environ Toxicol Chem 35(12):3058–3061.
- 12. Clarkson, C., Maharaj, V.J. Crouch, N.R., et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. (2004)J Ethnopharmacol 92(2–3):177–191.
- 13. Meyer, B.N., Ferrigni, N.R., Putnam, J.E., et al. Brine shrimp: a convenient general bioassay for active plant constituents. (1982) Planta Med 45(5):31–34.
- 14. Novoa, A.V., Mara de Oliveira Silva, A., Diaz Gutierrez, D., et al. Seaweeds from Halimeda Genus as Sources of Natural Antioxidants. (2017) J Anal Pharm Res 5(6): 00158.
- 15. Kim, H.P., Son, K.H., Chang, H.W., et al. Anti-inflammatory plant flavonoids and cellular action mechanisms. (2004) J Pharmacol Sci 96(3):229–245.
- 16. Vidal, A., De Silva Andrade-Wartha, E., de Oliviera e Silva, A., et al. Antioxidant activity and polyphenols of seaweed species Halimeda opuntia and Halimeda monile. (2009) ARS Pharm 50(1):24–31.
PubMed│CrossRef│Others
- 17. Sirinthipaporn, A., Jiraungkoorskul, K., Jiraungkoorskul, W. Artemia salina Lethality and Histopathological Studies of Siam Weed, Chromolaena odorata. (2017) J Nat Remedies 16(4):131.
- 18. Dosumu, O.O., Oluwaniyi, O.O., Oyedeji, O. Phytochemical Screening and Brine Shrimp Assay Investigation of Vegetables Commonly Consumed in Southern and North Central Parts of Nigeria. (2013) Centerpoint J 19(2):79–88.
PubMed│CrossRef│Others
- 19. Duffy, J.E., Hay, M.E. Seaweed Adaptations to Herbivory. (1990) Bioscience 40(5):368–375.
- 20. Paul, V.J., Van Alstyne, K.L. Chemical defense and chemical variation in some tropical Pacific species of Halimeda (Halimedaceae; Chlorophyta). (1988) Coral Reefs 6(3–4):263–269.
- 21. Aftab Uddin S. Seaweeds of Bangaldesh. (2019) Chittagong, Bangladesh: Institute of Marine Sciences, University of Chittagong 174 p.
PubMed│CrossRef│Others
- 22. Yamakami, Y., Morino, K., Takauji, Y., et al. Extract of Emblica officinalis enhances the growth of human keratinocytes in culture. (2019) J Integr Med 17(2):141–146.
- 23. Kim, S.J., Woo, S., Yun, H., et al. Total Phenolic Contents and Biological Activities of Korean Seaweed Extracts. (2005) Food Sci Biotechnol 14(6):798–802.
PubMed│CrossRef│Others
- 24. Gul, R., Jan, S.U., Faridullah, S., et al. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. (2017) Sci World J 2017:5873648.
- 25. Vimalkumar, C.S., Vilash, V., Krishnakumar N.M., et al. Comparative Preliminary Phytochemical Analysis of Ethanolic Extracts of Leaves of Olea Dioica Roxb, Infected with The Rust Fungus Zaghouania oleo(E.J Butler) Cummins and Non Infected Plants. (2014) J Pharmacogn Phytochem 3(4):69–72.
PubMed│CrossRef│Others
- 26. Ezeonu, C.S., Ejikeme, C.M. Qualitative and Quantitative Determination of Phytochemical Contents of Indigenous Nigerian Softwoods. (2016) New J Sci 2016: 1–9.
- 27. Dahanayake, J.M., Perera, P.K., Galappatty, P., et al. Comparative Phytochemical Analysis and Antioxidant Activities of Tamalakyadi Decoction with Its Modified Dosage Forms. (2019) Evidence-Based Complement Altern Med 2019: 6037137.
- 28. Das, B., Arpita, F., Morshed, M., et al. Phytochemical screening and Antioxidant activity of Leucas aspera. (2011) Int J Pharm Sci Res 2(7):1746–1752.
- 29. de Torre, M.P., Cavero, R.Y., Calvo, M.I., et al. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. (2019) Antioxidants8(5):142.
- 30. Lee, S., Min, B., Kho, Y. Brine shrimp lethality of the compounds from Phryma leptostachya L. (2002) Arch Pharm Res 25(5):652–654.
- 31. Morshed, M.A., Uddin, A., Rahman, A., et al. In vitro antimicrobial and cytotoxicity screening of Terminalia arjuna ethanol extract. (2011) Int J Biosci 1(2):31–38.
PubMed│CrossRef│Others
- 32. Morshed, M.A., Uddin, A., Saifur, R., et al. Evaluation of Antimicrobial and Cytotoxic Properties of Leucas aspera and Spilanthes paniculata. (2011) Int J Biosceinces 1(2):7–16.
PubMed│CrossRef│Others
- 33. Litchfield, J.T., Wilcoxon, F. A simplified method of evaluating dose-effect experiments. (1949) J Pharmacol Exp Ther 96(2):99–113.












