Primary Pancreatic Tuberculosis Mimicking Pancreatic Lymphoma in an Immunocompetent Patient: A Case Report
Ryan Kim , Vasanth Stalin , Samuel Shaheen
Affiliation
- 1Department of Surgery, Central Michigan University, USA
- 2Department of Surgery, University of Missouri-Columbia, USA
- 3Ross University School of Medicine, Dominica
Corresponding Author
Kent Wright, Ross University School of Medicine, Dominica E-mail: kentwright@students.rossu.edu
Citation
Wright, K., et al. Primary Pancreatic Tuberculosis Mimicking Pancreatic Lymphoma in an Immunocompetent Patient: A Case Report. (2016) J Gastro Dis Liver Func 2(1): 52- 55.
Copy rights
© 2016 Wright, K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
The medical discovery of a pancreatic mass has traditionally been associated with a short list of differential diagnosis most often representing an ominous prognosis of pancreatic malignancy. Historically, the possibility of pancreatic tuberculosis (PTB) has not been considered because the medical consensus, as stated by Hari et al[1] and Singh et al[2] believed that pancreatic enzymes were inhibitory to the seeding of tubercle bacilli. Our clinical experience agrees with recent scientific literature showing primary pancreatic manifestation of Mycobacterium Tuberculosis (MTB) do occur and presents as a solitary lesion with multiple cystic components in the head, body or tail of the pancreas. The physiological presentation, as described by Rana et al[3] and Sahani et al[4] mimics 90% of primary cystic pancreatic tumors covered by the three entities, serous or mucinous cystadenoma and intraductal papillary mucinous neoplasm. Its gross appearance is indistinguishable from the pancreatic neoplasms. Literature by both Ladas et al[5] and Hong et al[6] agree that PTB displays no radiographic features which are pathognomonic for its diagnosis. Despite the atypical presentation, scientific literature suggests Mycobacterium possesses the molecular capability for a wide variety of infectious mechanisms including primary PTB. This is a curable disease which warrants clinical investigation and should be included in the list of differentials to prevent erroneous diagnosis of pancreatic malignancy and the unnecessary morbidity of surgery.
Introduction
Tuberculosis continues to be one of the leading infectious diseases worldwide affecting one third of the world’s population with an annual incidence of 9.6 million people in 2014. During the same time period 9,421 new cases were reported in the United States of which 66.5 percent were foreign born persons. Pulmonary infections represent the majority of the cases but TB can infect any organ within body. However, the extra-pulmonary infections have been thought to occur primarily in immunocompromised individuals. Historically, the possibility of pancreatic tuberculosis (PTB) has not been considered in immunocompetent individuals because the pancreatic enzymes were thought to inhibit the seeding of tubercle bacilli[1,2]. PTB is often overlooked and not considered as a plausible pathology for a pancreatic mass due to the following factors. The pancreatic manifestation is extremely rare in the absences of pulmonary tuberculosis. The presentation lacks pathognomonic features which distinguish it from malignancy both radiologically or on physical presentation[7]. Additionally, confirmation of this diagnosis is slow, requiring microbiological growth which can take six weeks or more to culture. Several diagnostic techniques have been proposed, namely EUS, MRI, PET, CT, FNA, and laparotomy. Our patient had more than one issue in his abdomen that required surgery but pancreatic tuberculosis on its own is a disease that can be treated medically, so proper diagnosis can be one that saves a patient from unnecessary surgery. In this report we present the case of primary pancreatic tuberculosis mimicking as pancreatic lymphoma in 20 year old immunocompetent male.
Case Report
Our Patient was a 20 year old Nepalese male, who immigrant to the United States 4 years earlier. He presented with abdominal pain of 2 months duration which he described as a consistent, sharp, epigastric pain that radiated to the chest with ingestion of food. He reported a 20 pound weight loss over the past 2 months, night sweats, fever, and chills, but denied any nausea, vomiting, jaundice, cough, hemoptysis or shortness of breath. He had no significant past medical history and his immunizations were all up to date, including the BCG vaccine received at childhood. His physical examination was unremarkable, except for mild epigastric tenderness.
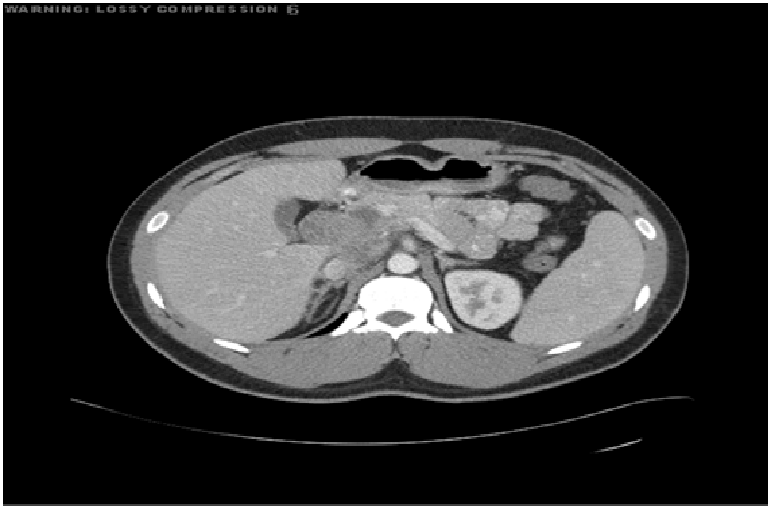
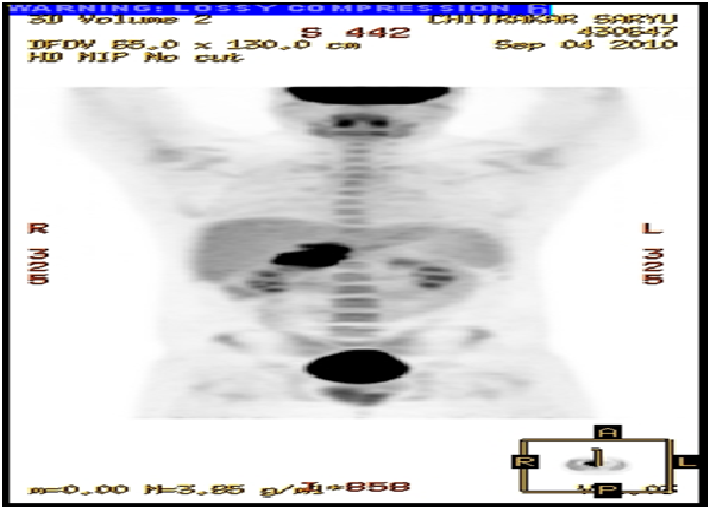
Prior to being referred to our general surgery clinic he underwent many diagnostic studies: His blood test—Complete blood count, Basic metabolic panel, Liver function test, Lipase, and Amylase—were all within normal limits. The esophagogastroduodenoscopy (EGD) showed only a small ulcer in the prepyloric antrum, however, the initial computed tomography (CT) revealed an ill-defined lobulated mass (44 × 59 × 56 mm) in the retro and peripancreatic region extending into the hepatic hilum with encasement of the proximal portal vein and hepatic artery without dilatation of the biliary tract or pancreatic duct, and with periaortic lymphadenopathy (Figure 1). Subsequently he underwent CT guided biopsy, which showed reactive lymphoid parenchyma with heterogeneous population of lymphocytes, histiocytes and histiocytic tangles, and some necrotic material. He then underwent Positron emission tomography (PET) which showed a large mass, involving the head of the pancreas, the uncinate process of the pancreas and the caudate lobe of the liver, with standardized uptake value maximum of 13.1 (Figure 2). With these findings, a leading differential diagnosis of pancreatic lymphoma was made and he was referred to our general surgery clinic for definitive tissue biopsy diagnosis, and possible surgical interventions.
Figure 1: Preoperative CT showing an ill-defined lobulated mass (44 X 59 X 56 mm) in the retro and peripancreatic region extending into the hepatic hilpum with encasement of the proximal portal vein and hepatic artery in addition to periaortic lymphadenopathy. No dilatation of the biliary tract or pancreatic duct is seen.
Figure 2: Preoperative PET scan showing a large mass, involving the head of the pancreas, the uncinate process of the pancreas and the caudate lobe of the liver.
After discussing with the patient and the family, we elected to proceed with diagnostic laparoscopy with pancreatic biopsy. Intraoperatively, three 5mm laparoscopic ports were utilized in the supraumbilical, subxiphoid and left upper quadrant region. By incising the gastrocolic ligament, the transverse colon was mobilized and it allowed exposing the head of the pancreas. Multiple biopsies from the pancreas were taken with 5 mm biopsy forceps as well as the Harmonic scalpel and sent for frozen section evaluation, however, the results were benign (benign pancreatic tissue, focal fibrosis and mucinous material, necrotic tissue with acute and chronic inflammation, and fibrous tissue with acute and chronic inflammation). Without the definitive diagnosis, we then converted to open laparotomy. An extensive Kocher maneuver was performed, and the whole pancreas was inspected and we appreciated a large hard mass in the superior aspect of the pancreatic head. While debriding the necrotic pancreatic tissue in this area, an abscess cavity was entered posteriorly to the duodenum and common bile duct. Multiple specimens were again sent for frozen section evaluation, and there was no malignancy identified. Multiple culture swabs of the pus and tissues were sent for microbiology. Further debridement of the pancreas was performed and Jackson-Pratt drain was placed in the pancreatic abscess cavity. The liver was found to be smooth and free of any gross abnormalities, and no other irregularities identified in the abdominal cavity. The final pathology report of the surgical specimen showed pancreatic tissue with extensive necrosis, mixed acute and chronic inflammation, and focal granuloma reaction. The special staining of GMS and PAS for fungus and AFB for mycobacteria result was negative. HIV, Hepatitis viral panel, Immunoglobulin level were all normal.
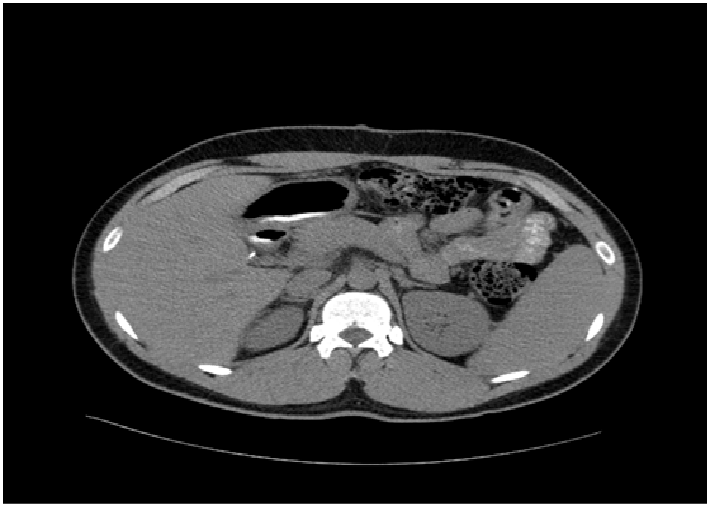
His postoperative course was uneventful and he was discharged home on postoperative day 10. However, the acid/fast culture became positive on day 44, and he was immediately started on Isoniazid, Rifampin, Ethambutol and Pyrazinamide. The organism was identified as Mycobacterium tuberculosis complex via High performance Liquid Chromatography, and it was susceptible to Isoniazid, Rifampin, Ethambutol, and Pyrazinamide. One month after being on all 4 medications, Ethambutol and Pyrazinamide were discontinued. Patient did well with the antitubercular drugs and 5 month follow up CT showed a significant improvement in the pancreatic region (figure 3). The antibubercular therapy was stopped at 6 month, and he was doing well at the one-year follow up.
Figure 3: Follow up CT at 5 months showed a significant improvement in the pancreatic region
Discussion
More than 120 years have passed since Robert Koch established a link between MTB and its pathology, yet the complexity and versatility of its infectious mechanisms are still not fully understood. Upon review of literature, it becomes clear that about 1.7 billion people worldwide are infected with Mycobacterium tuberculosis, causing both pulmonary and disseminated disease. The mechanism of seeding for pancreatic tuberculosis is thought to be either hematogenous or lymphatic spread from peripancreatic lymph nodes. Patients with Pancreatic tuberculosis often seem to present with nonspecific symptoms, without a history of tuberculosis or pancreatic disease. The majority of these patients have no history of pulmonary tuberculosis, suggesting latent reactivation of a pancreatic tuberculous focus months to years after the initial disseminated primary infection. In 1944 Auerbach revealed multiple variations in the MTB pathology. His study of 297 miliary TB autopsies showed pancreatic involvement in 4.7% of cases[1]. More recently, Chao et al[8] has shown the versatility of MTB, identifying numerous signaling pathways. Their research has shown a molecular system which provides a means for adaptive gene expression and regulation of metabolic processes in response to external stimuli, dictating the course of the infection. Furthermore, DNA fingerprinting since the 1990s has identified 10,000s of different Mycobacterium serotypes[9]. Combined, this is enough evidence to suggest MTB contains the ability for multiple unlikely pathologies including primary infection of the pancreas of immunocompetent individuals.
A manifestation in the pancreas in the absence of TB in any other organ is an extremely rare occurrence and currently has no explanation for its evolution[10]. However, there is increasing evidence of primary PTB. Nager et al. state nearly 100 cases reported worldwide by 2009[11,12]. This reflects an increased global incidence of nearly 5 cases annually from the 41 cases recorded during 1966-1997[5]. The understood course of extra-pulmonary infection suggests tubercle bacilli enter the host through inhalation where they hijack macrophage cells and replicate within the endosomal compartment. If spreading ensues from the primary infection it will occur via the lymphatics to the regional lymph nodes then eventually to the blood stream where it may spread to any other organ[13,14]. The most common affected organs include the abdominal lymph nodes, small intestine, cecum, liver, spleen and peritoneum. Alternatively, TB can infect the GI by ingestion of milk contaminated with Mycobacterium Bovis. These infections can similarly spread to other organs via the celiac and retroperitoneal lymph nodes[15]. Although pasteurization has made this rare in developed countries, it can still occur in regions of the world where raw milk is regularly consumed. A Mycobacterium infection of the pancreas presents as a cystic mass which is difficult to distinguish from malignancy and is often not diagnosed until after laparotomy. Literature from Schneider et al[10] and Saluja et al[16] reported 31/41 and 4/4 cases, respectively of post-operative diagnosis. Once identified the prognosis of PTB is good with only 4 out of 73 cases reported in literature resulting in death from the disease[17,18].
The diagnosis of PTB requires histopathological or microbiological confirmation which is best obtained through image guided FNAC[1,19]. The confirmatory microscopic features of PTB are caseation necrosis and acid fast bacilli[2]. The caseating granuloma is seen 75 - 95% of the cases and acid fast bacilli are identified 20 - 40% of the cases. It should be noted that Mycobacteria are difficult to culture and may require up to 6 weeks for growth[20,21]. This process can be expedited with PCR which provides same day results with a 77% sensitivity[2,15]. Discovery of caseating granuloma or acid fast bacilli should provide a high level of confidence in the diagnosis since there are currently no documented cases of PTB coexisting with pancreatic malignancies[2].
Conclusion
More than a century of research has established Mycobacterium Tuberculosis to be a complex pathogen. Its molecular response to external stimuli provides a diversity of mechanisms for infecting multiple organs individually or simultaneously. A primary manifestation of the pancreas is extremely atypical, presenting with an annual incidence of fewer than 5 cases per year. This course of infection is not fully understood and there are currently no pathognomonic features in the physical or radiological presentation to allow for easy identification. Therefore it may not be considered as a differential in patients presenting with a pancreatic mass. The evidence of our case and recent scientific literature shows TB infection of the pancreas do occur and are a plausible cause of cystic pancreatic masses. Any patient presenting with a cystic mass in the pancreas should be considered to have cancer until proven otherwise; however, the primary pancreatic tuberculosis infection may mimic malignancy by its vague symptoms and nonspecific findings. Unfortunately, with the preoperative diagnosis of malignancy many patients undergo extensive surgical intervention.
Therefore, primary tuberculosis should be considered as a possible differential, even in the absence of TB from the rest of the body, especially, if they have a history of travel through endemic regions. Careful pathologic examination and microbiology cultures of the specimen are the key to a successful diagnosis, reducing erroneous diagnosis of pancreatic malignancy and the incidence of unnecessary surgery. This is a curable disease which responds favorably to antituberculotic therapy, typically resulting with complete resolution of the disease.
References
- 1. Hari, S., Seith, A., Srivastava, D.N., et al. Isolated Tuberculosis of the Pancreas Diagnosed With Needle Aspiration. (2005) Trop Gastroenterol 26(3): 141-143.
- 2. Singh, D.K., Haider, A., Tatke, M., et al. Primary Pancreatic Tuberculosis Masquerading as a Pancreatic Tumor Leading to Whipple's Pancreaticoduodenectomy: A Case Report and Review of the Literature. (2009) JOP 10(4): 451-456.
- 3. Rana, S.S., Bhasin, D.K., Rao, C., et al. Isolated pancreatic Tuberculosis Mimicking Focal Pancreatitis and Causing Segmental Portal Hypertension. (2010) JOP 11(4): 393-395.
- 4. Sahani, D.V., Kadavigere, R., Saokar, A., et al. Cystic Pancreatic Lesions: A Simple Imaging-Based Classification System for Guiding Management. (2005) Radiographics 25(6): 1471-1484.
- 5. Lada, S.D., Vaidakis, E., Lariou, C., et al. Pancreatic Tuberculosis in Non-Immunocompromised Patients: Report of Two Cases and a Literature Review: (1998) Eur J Gastroenterol Hepatol 10(11): 973-997.
- 6. Hong, S.G., Kim, J.S., Joo, M.K., et al. Pancreatic Tuberculosis Masquerading as Pancreatic Serous Cystadenoma. (2009) World J Gastroenterol 15(8): 1010-1013.
- 7. Khaniya, S., Koirala, R., Shakya, V.C., et al. Isolated pancreatic tuberculosis mimicking as carcinoma: a case report and review of the literature. (2010) Cases J 3: 18.
- 8. Chao, J., Wong, D., Zheng, X., et al. Protein Kinase and Phosphatase Signaling in Mycobacterium Tuberculosis Physiology and Pathogenesis. (2010) Biochim Biophys Acta 1804(3): 620-627.
- 9. Dormans, J., Burger, M., Aguilar, D., et al. Correlation of Virulence, Lung Pathology, Bacterial Load and Delayed Type Hypersensitivity Response after Infection with Different Mycobacterium Tuberculosis Genotypes in a BALB/c Mouse Model. (2004) Clin Exp Immunol 137(3): 460-468.
- 10. Schneider, A., Birgelen, C., Duhrsen, U., et al. Two Cases of Tuberculosis in Non immunocompromised patients: A Diagnostic Challenge and a Rare Cause of Portal Hypertension. (2002) Pancreatology 2(1): 69-72.
- 11. Anna, Falkowski., Judith, Graber., Horst, G., et al. Isolated Pancreatic Tuberculosis: A Case Report and Radiological Comparison with Cystic Pancreatic Lesions. (2013) J Radiol Case Rep 7(1): 1-11.
- 12. Nagar, A.M., Raut, A.A., Morani, A.C., et al. Pancreatic Tuberculosis: A Clinical and Imaging Review of 32 Cases. (2009) J Comput Assist Tomogr 33(1): 136-141.
- 13. Jawetz., Melnick., Adelberg. Mycobacteria Chapter 23. Medical Microbiology 26e.
- 14. Demir, K., Kaymakoglue, S., Besisik, F., et al. Solitary pancreatic Tuberculosis in Immunocompetent Patients Mimicking Pancreatic Carcinoma. (2001) J Gastroenterol Hepatol 16(9): 1071-1074.
- 15. Xia, F., Poon, R.T.P., Wang, S.G., et al. Tuberculosis of Pancreas and Peripancreatic Lymph Nodes in Immunocompetent Patients: Experience from China. (2003) World J Gastroenterol 9(6): 1361-1364.
- 16. Saluja, S.S., Ray, S., Pal, S., et al. Hepatobiliary and Pancreatic Tuberculosis: A Two Decade Experience. (2007) BMC Surg 7:10.
- 17. Dorhoi, A., Reece, S., Kaufmann, S. For Better or for Worse: The Immune response against Mycobacteria Tuberculosis Balances Pathology and protection. (2011) Immunol Rev 240(1): 235-251.
- 18. Foo, F.J., Verbeke, C.S., Guthrie, J.A., et al. Pancreatic and Peripancreatic Tuberculosis Mimicking Malignancy. (2007) JOP 8(2): 201-205.
- 19. Loya, A.C., Prayaga, A.K., Sundaram, C., et al. Cytologic Diagnosis of Pancreatic Tuberculosis in Immunocompetent and Immunocompromised Patients. (2005) Acta Cytol 49(1): 97-100.
- 20. Ray, S., Das, K., Mridha, A.R. Pancreatic and Peripancreatic Nodal Tuberculosis in Immunocompetent Patients: Report of Three Cases. (2012) JOP 13(6): 667-670.
- 21. Yavuz, A., Bulu, H., Aydin, A., et al. Pancreatic Tuberculosis Mimicking Inoperable pancreatic Cancer. (2012) Turk J Gastroenterol 23(1): 95-97.