Protection Against Dimethylbenz[a] Anthracene-Induced Breast Cancer in Female Rats by alpha-Lactalbumin
Somdutta Sinha Roy1, Shyamali Mukherjee2, Billy R Ballard3, Salil K Das1*
Affiliation
- 1Department of Biochemistry & Cancer Biology, Meharry Medical College, Nashville, TN, USA
- 2Department of Professional Education, Neurosciences & Pharmacology, Nashville, TN, USA
- 3Department of Pathology, Meharry Medical College, Nashville, TN, USA
Corresponding Author
Salil K. Das, Sc.D., D.Sc, Professor of Biochemistry & Cancer Biology, Meharry Medical College, Nashville, TN 37208, USA. Tel: (615) 327-6988; Fax: (615) 327-6442; E-mail: sdas@mmc.edu
Citation
Das, S.K., et al. Protection Against Dimethylbenz[a] Anthracene-Induced Breast Cancer in Female Rats by α-Lactalbumin. (2016) Intl J Cancer Oncol 3(1): 1-6.
Copy rights
© 2016 Das, S.K. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Breast cancer; Rat; alpha-lactalbumin; Casein; Proliferative index; Pathology
Abstract
Consumption of α-lactalbumin as dietary protein offers a beneficial effect on breast cancer development. Breast cancer was developed by gavage administration of single dose of dimethylbenz(a)anthracene (DMBA) in female rats, maintained on AIN-76A diet with either 20% casein or α-lactalbumin (a component of whey protein). All tumors were detected by palpation. After approximately 130 days of DMBA administration, the animals were euthanized. There was a delay in the development of breast tumor in the α-lactalbumin group in comparison to the casein group. The number of tumors per rat was less in the α-lactalbumin group than that in the casein group at any time point up to 130 days after DMBA administration. Also the incidence of tumors and tumor volume was less in the α-lactalbumin group than those in the casein group. The casein group had a mixture of grade I, grade II and grade III tumors whereas the α-lactalbumin group had mostly grade I tumor. Furthermore, the proliferative index was significantly lower in the α-lactalbumin group than that in the casein group.
Introduction
Breast cancer is the second leading cause of death of women (after lung cancer) in North American and European countries. In USA, 135,000 new cases of breast cancer are reported each year[1]. Of all the factors known to influence breast cancer, diet appears to be one of the most significant[2]. Milk and dairy products are important part of the western style diet and are known to exert inhibitory effects on the growth of certain tumor types[3]. An anti-tumor activity of the dairy products has been attributed to a class of proteins that represent 20% of the total milk protein, the whey fraction[4].
The active components of whey include lactoferrin, ß-lactoglobulin, α-lactalbumin, serum albumin, glycomacropeptide, lactoperoxidase enzymes, lactose, minerals and immunoglobulins[5]. Furthermore, others have demonstrated that whey is particularly rich in substrates for synthesis of glutathione (GSH) and responsible for increased GSH concentration in a number of tissues[6]. Collectively, whey proteins have all the essential amino acids and in higher concentrations compared to various vegetable protein sources such as soy, corn, and wheat gluten[7]. Additionally, the amino acids found in whey are efficiently absorbed and utilized when compared to free amino acid solutions[8]. Whey protein contains 8 times more of the amino acid cysteine than casein[9].
Several studies have shown that dietary whey protein has a protective effect on various types of cancer, like azoxymethane-induced colon tumors[10] and 7, 12-dimethylbenz (a) anthracene (DMBA)-induced mammary tumors in rats[11]. Even though these studies could show a beneficial effect of dietary whey protein in reducing both tumor incidence and multiplicity, no histopathology of tumor type and gradation was done and also no mechanism was elucidated. Subsequently it has been shown that substitution of whey protein isolate by α-lactalbumin does not affect the protein quality or mineral availability in rats[12] and many subsequent studies concentrated on α-lactalbumin.
α-lactalbumin is a small (14,200 mw), acidic, Ca2+-binding protein. It is one of the two proteins comprising the lactose synthase enzyme complex that catalyses the final step of lactose synthesis which occurs in the Golgi lumen of the lactating mammary gland. α-lactalbumin is then secreted as a component of milk, where it may serve other purposes[13]. Interest in α-lactalbumin has grown in past several years as α-lactalbumin itself or its fragments have shown to possess bactericidal or antitumor activity[14,15]. It has been shown that a multimeric α-lactalbumin derivative (MAL) is a potent Ca2+ elevating and apoptosis inducing agent with broad, yet selective, cytotoxic effect[14]. It was shown to kill all transformed, embryonic, and lymphoid cells but spare the matured cells. A lot of studies have also been done on α-lactalbumin complexed with oleic acid (HAMLET: human α-lactalbumin made lethal to tumor cells or BAMLET: bovine α-lactalbumin made lethal to tumor cells). More recently however, it has been shown that monomeric α-lactalbumin shows similar interactions with histones and basic poly-amino acids (poly-lys, poly-arg) as HAMLET or BAMLET[16].
Mechanism for apoptosis induction by α-lactalbumin or its variants have been shown to be through release of cytochrome c from mitochondria and activation of caspase cascade[17-19]. Whey has been shown to act by down-regulating DMBA-induced CYP1 A1 and CYP1 B1 expression[20], as these enzymes are responsible for conversion of pro-carcinogen DMBA to its ultimate carcinogenic metabolite, DMBA-3,4-dihydrodiol-1,2-epoxide. Whey has also shown to increase progesterone receptor (PR) expression which is not an estrogenic effect but may be due to peptides generated during processing, or growth factors found in whey[21]. Considering these facts the present study was conducted to confirm the possible preventive effects of α-lactalbumin in diet on the development of breast cancer in rats. The aim of this study was to determine whether dietary α-lactalbumin has any effect on tumor incidence, volume of tumor, as well as tumor grade in female rats. After validation of this model, our next goal will be to elucidate the mechanism by which α-lactalbumin exerts its anti-tumor activity.
Materials and Methods
Development of breast tumor in female rats
Adult female Sprague Dawley rats were purchased at 22 days of age from Harlan Sprague-Dawley, Inc. Indianapolis, IN). They were housed individually in polycarbonate cages. Animals were divided into four groups. Each group contained 10 animals. Animals from groups 1 and 2 were fed with standard AIN-76A diet containing 20% casein and those of groups 3 and 4 were fed with same diet containing 20% α-lactalbumin instead of 20% casein as a form of pellet. The diets were prepared by Harlan Teklad (Madison, WI) and the composition of diets is shown in Table 1.
Table 1: Composition of diets
| Ingredients | Casein (g/kg) | α-lactalbumin (g/kg) |
|---|---|---|
| Casein | 200.0 | - |
| α-lactalbumin | - | 200.0 |
| L-Cystine | 3.68 | 1.1 |
| DL-Methionine | - | 0.3 |
| Sucrose | 482.7273 | 485.6873 |
| Corn Starch | 150.0 | 150.0 |
| Corn Oil | 50.0 | 48.0 |
| Cellulose | 50.0 | 50.0 |
| Mineral Mix, AIN-76 (170915) | 35.0 | 35.0 |
| Calcium Carbonate | 9.68 | 6.0 |
| Calcium Phosphate, dibasic | - | 5.0 |
| Cupric Carbonate | 0.0057 | 0.0057 |
| Ferric Citrate | 0.156 | 0.156 |
| Sodium Bicarbonate | 6.124 | 6.124 |
| Vitamin Mix, AIN-76A (40077) | 10.0 | 10.0 |
| Choline Bitartrate | 2.617 | 2.617 |
| Ethoxyquin (antioxidant) | 0.01 | 0.01 |
The animals were placed on the test diets at 25 days of age and remained on the diet for the rest of the study. Rats were allowed to feed and drink water ad libitum.
Mammary tumors were induced in rats of groups 2 and 4 by a single dose of intragastric administration of a carcinogen, DMBA (Sigma Chemical Co, St Louis, MO) in sesame oil (80 mg/kg b.wt) at 50 days of age. Control animals (groups 1 and 3) received the vehicle only by gavage.
Animals were weighed and also palpated twice weekly to detect tumors beginning four weeks after the administration of carcinogen. At 130 days post-administration of DMBA, animals were sacrificed by CO2 asphyxiation. All tumors were weighed and measured for volume, and a section of the tumor was fixed in buffered formalin. Sections of the paraffin-embedded tumors were stained with hematoxylin (H) and eosin (E) for histological analysis. The remaining tissues were stored at -80°C for biochemical studies.
Histological grading of mammary gland adenocarcinoma
A portion of breast tissue from all animals were fixed overnight in neutral buffered formalin, pH 7.5, and embedded in paraffin. Five microns sections were stained with H & E for light microscopic studies. The slides were examined and scored by a certified pathologist as described earlier[1]. The final grade was determined by the total score based on the tubule formation within the neoplasm, nuclear pleomorphism, and mitotic count per 10 high power field (hpf).
Tubule formation was given a score of 1 when an overall evaluation of the tumor showed formation of tubules with visible lumens in a majority (> 75%) of the lesion. When solid areas of tumor growth were admixed with a moderate degree of the tubular arrangements (10 - 75%), the tumor was given a score of 2. When the tumor showed minimal or no tubule formation (< 10%), it received 3 points.
In the assessment of nuclear pleomorphism, variation in the size and shape of the tumor nuclei was evaluated. A score of 1 was given to tumors with uniform or regular, small nuclei and those exhibiting minimal variation. Tumors with a moderate degree of variation in nuclear size and shape and occasional nuclei were given 2 points. Those with marked variation in nuclear size and containing bizarre nuclei, often with one or more prominent nucleoli, were given 3 points.
In determination of the mitotic rate, at least 10 fields in the periphery or the most mitotically active part of the tumor were evaluated. The tumor was scored 1 - 3 depending on the number of mitotic figures per 10 hpf.
Using the above parameters mammary gland adenocarcinomas were graded I, II, and III. Grade I was the least aggressive and had the best prognosis and grade III was the most aggressive and had the worst prognosis. Grade I had a total score between 3 and 5, grade II had a total score between 6 and 7; and grade III had a total score between 8 and 9.
Immunohistochemical staining of Ki-67 for assessment of mitotic activity
Formalin (10%) fixed sections were incubated with 0.3% H2O2 to block endogenous peroxide activity. The sections were washed and incubated with normal rabbit serum for 30 min to block non-specific binding. Afterwards, the sections were incubated with a polyclonal antibody for Ki-67 (diluted 1/50 in PBS, DAKO). The sections were washed and then incubated with biotinylated secondary antibody for 1 h. The sections were washed and incubated with HRP-Streptavidin for 1h at room temperature and reacted with diaminobenzamidine until reddish brown color developed. Sections were then counterstained with Gill’s hematoxylin solution, and cover slipped. Ki-67 reactivity was evaluated by counting the number of positive and negative epithelial cell nuclei in several randomly selected fields in each section[22].
Statistical Analyses
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Inc., San Diego, CA). Comparisons were made between groups using the paired two-tailed Student’s t-test and a p value of < 0.05 was considered significant.
Results
Body weights
The body weight gains were similar in all four groups. Neither DMBA administration nor the nature of dietary protein had any significant effects on the body weight gains of the animals (data not shown).
Time course for tumor formation
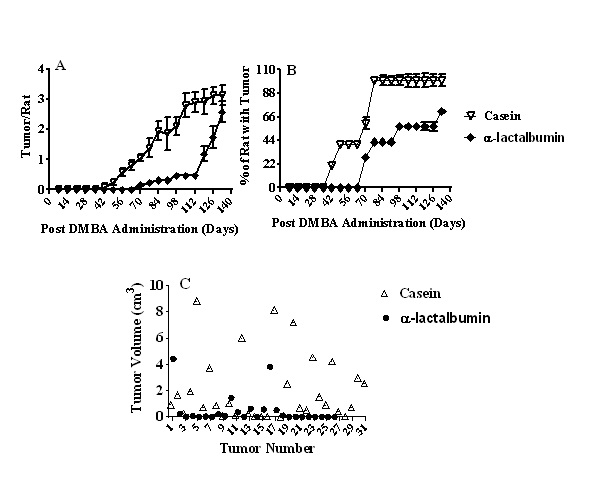
The time course of palpatable breast tumor development is shown in Figure 1A. Replacement of casein by α-lactalbumin caused a significant delay in the initiation of breast tumor development (42 day for casein vs 62 day for α-lactalbumin). Furthermore, although multiple tumors were observed in animals of both groups, the number of tumors per rat was significantly less (p < 0.001) in the α -lactalbumin group than that in the casein group at any time period (day 42 to day 130) after DMBA administration.
Figure 1: A: Time course of palpatable breast tumors from rats fed either casein or α-lactalbumin and treated with DMBA. Each group contained 10 animals. Results are expressed as mean ± SEM. Significantly different between the groups up to 80 days after DMBA administration, p < 0.001;
B: Breast tumor incidence from rats fed either casein or α-lactalbumin and treated with DMBA. Each group contained 10 animals. Results are expressed as mean ± SEM. Significantly different between the groups up to 80 days after DMBA administration, p < 0.001;
C: Tumor volume of rats fed either casein or α-lactalbumin and treated with DMBA. Tumor volume was significantly higher in the casein group in comparison to the α-lactalbumin group (p < 0.005).
Breast tumor incidence
Breast tumor incidence (percentage of rats with tumors) is shown in Figure 1B. The incidence of tumors was significantly less (p < 0.001) in the α-lactalbumin group than that in the casein group at any time period (day 42 to day 130) after DMBA administration.
Tumor characteristics
There was no tumor in any animal, which did not receive the carcinogen regardless of whether they were fed casein or α-lactalbumin as protein in the diet. Even though there was a difference in the time course of tumor development between the two dietary groups, the tumors were visibly apparent externally for both groups. Data on tumor volume are shown in Figure 1C. There were 31 tumors in the casein group and 26 tumors in the α-lactalbumin group. The tumor volume was significantly larger (p < 0.005) in the casein group (2.04 ± 0.45 cm³) than that in the α-lactalbumin group (0.48 ± 0.21 cm³).
Out of 31 tumors in the casein group, 14 were of larger size (> 1 cm³) and 17 were of smaller size (< 1 cm³). However, in the α-lactalbumin group, out of 26 tumors, only 3 were of larger size (> 1 cm³) and 23 were of smaller size (< 1 cm³).
Pathology of breast tumors
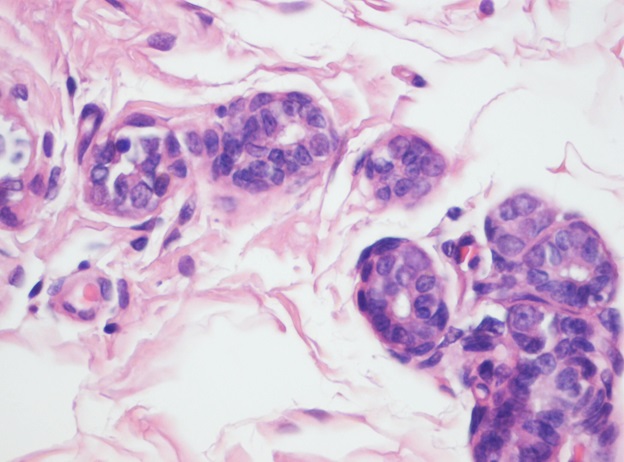
Representative photograph of light microscopy from breast tissue of control animals regardless of whether they were fed casein or α-lactalbumin is shown in Figure 2. It reveals scattered normal lobuloalveolar units lined by a layer of cuboidal epithelium and a discontinuous layer of flattened myoepithelial cells and terminal end buds in an adipose tissue stroma (H & E x100). No tumor was noticed in any animal.
Figure 2: Histological section from breast of rats fed either casein or α-lactalbumin without treatment with DMBA (H & E X100). No tumor was found in any group and they had same morphology.
As shown in Table 2, a higher percentage of aggressive tumors was found in the DMBA/casein group (20% grade I, 60% grade II and 20% grade III), whereas 100% of the mammary gland adenocarcinoma found in the DMBA/α-lactalbumin group was of the non-aggressive type (grade I).
Table 2: Effect of diets on histological grading of mammary gland adenocarcinoma in female rats-induced by DMBA
| Dietary Protein | No of Specimens Examined | Tumor Grade | |||
|---|---|---|---|---|---|
| Grade 0 | Grade I | Grade II | Grade III | ||
| Casein | 20 | 1 | 4 | 11 | 4 |
| α-lactalbumin | 22 | 8 | 14 | 0 | 0 |
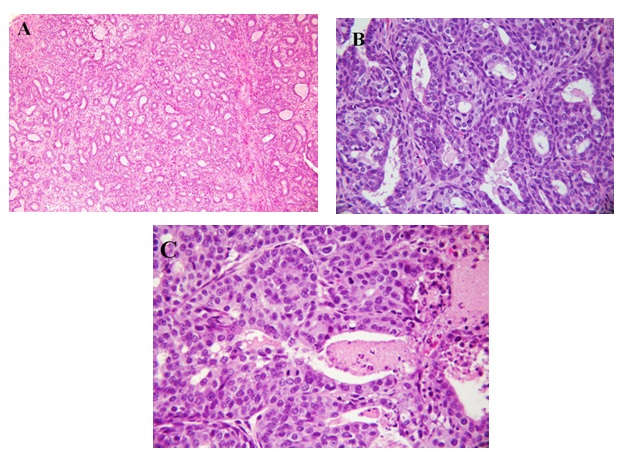
Representative photographs of light microscopic structure of breast tumors found in the DMBA/casein group are shown in Figure 3 [A: grade I, B: grade II, C: grade III].
Figure 3: A: Histological section from breast of rats fed casein and treated with DMBA showing a grade I modified Scarff-Bloom-Richardson mammary duct carcinoma (H & E X100);
B: Histological section from breast of rats fed casein and treated with DMBA showing a grade II modified Scarff-Bloom-Richardson mammary duct carcinoma (H & E X400);
C: Histological section from breast of rats fed casein and treated with DMBA showing a grade III modified Scarff-Bloom-Richardson mammary duct carcinoma (H & E X400).
According to Figure 3A, the tumor is composed of cells arranged in isolated small round to oval to elongated tubules lined by columnar epithelial cells with one to two layers of basal nuclei. The lining epithelial cells exhibit enlarged, crowded, uniform, hyperchromatic nuclei with evenly disbursed finely granular chromatin and indistinct nucleoli. The basal nuclei of the tubular epithelium show an absence of myoepithelial cells, indicating infiltrating neoplastic cells. The tubules are mostly isolated, widely spaced, and surrounded by a copious amount of connective tissue, occasional tubules show little intervening stroma and merging of the tubules. The solid areas displayed in the lesion are less than 25%. This lesion is comparable to a lowgrade tumor, grade I modified Scarff-Brown-Richardson mammary duct carcinoma (H & E x 100).
Figure 3B shows grade II modified Scarff-Bloom-Richardson mammary duct carcinoma. This tumor is of groups of tumor cells in a circumscribed and nodular pattern and composed sheets of proliferating tumor cells with tubular elements comprising 50% of the tumor cells. The nuclei are crowded, enlarged, slightly pleomorphic, with dense finely to coarsely granular chromatin, and visible nucleoli. Ten mitoses per 10 hpf at x 400 are present in this among few of the animals. Areas of tumor necrosis and acute and chronic inflammation are not identified.
Figure 3C shows grade III modified Scarff-Bloom-Richardson mammary duct carcinoma. This tumor is composed of sheets of infiltrating tumor without tubular elements comprising more than 75% of the tumor cells arranged in solid structures. The nuclei are crowded, enlarged, pleomorphic, with dense finely to coarsely granular chromatin, and visible nucleoli. More than 20 mitoses per 10 hpf at x 400 are present in this animal. Areas of tumor necrosis with polymorphonuclear neutrophil, lymphocyte, and plasma cell infiltrates are present.
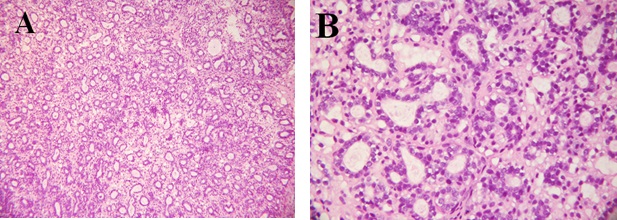
Representative photograph of light microscopic structure of breast tumors found in the DMBA/α-lactalbumin group are shown in Figure 4 (A: H & E x100, B: H & E x 400). It reveals that the tumors are composed of cells arranged in isolated small round to oval elongated tubules lined by columnar epithelial cells with one or two layers of basal nuclei. The lining epithelial cells exhibit enlarged, crowded, uniform, hyperchromatic nuclei with evenly disbursed finely granular chromatin and indistinct nucleoli. The basal nuclei of the tubular epithelium show an absence of myoepithelial cells, indicating infiltrating neoplastic cells. The tubules vary in size and shape, are mostly isolated, widely spaced, and surrounded by a copious amount of fibrous connective tissue. Occasional tubules show little intervening stroma. The solid areas displayed in the lesion are less than 25% of the lesion. This lesion is comparable to a low-grade tumor, grade I modified Scarff-Bloom-Richardson mammary duct carcinoma.
Figure 4: Histological section from breast of rats fed α-lactalbumin and treated with DMBA showing a grade I modified Scarff-Bloom-Richardson mammary duct carcinoma (A: H & E X 100; B: H & E x 400).
Immunohistochemical staining of Ki-67 for assessment of mitotic activity
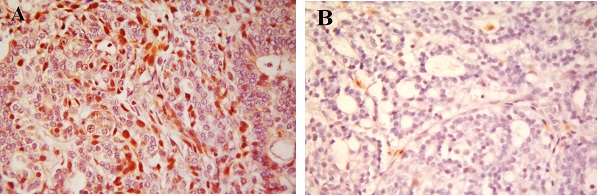
The mitotic activity assessed in grading the tumors was verified by immunohistochemical reactions for Ki-67 antibody. Tumors from animals in the DMBA/casein group had more than 50% of the cells staining positive with Ki-67 antibody (Figure 5A). However, tumors from the animals of the DMBA/α-lactalbumin group showed less than 10% of the cells staining with Ki-67 (Figure 5B). Thus, proliferative index was significantly lower in the α-lactalbumin group than that in the casein group.
Figure 5: A: Ki-67 lebeling index. Immunohistochemical section from breast of rats fed casein and treated with DMBA (x 400);
B: Ki-67 lebeling index. Immunohistochemical section from breast of rats fed α-lactalbumin and treated with DMBA (x 400).
Discussion
Cancer rates in countries with high consumption of soybeans and whey are lower than those in the United States[1], and these rates increase in second generation of the families that are migrated to the United States and their diets become Westernized[23]. We previously reported that soy protein may protect against the development of a more aggressive breast carcinoma[1]. Data from the present study substantiate the breast cancer protection claims for experimental diet containing whey protein isolate as reported by others[11,24]. Furthermore, the present data extend our knowledge on α-lactalbumin containing diet and add new information on its effect on initiation, multiplication, volume and aggressiveness with respect to chemically induced breast cancer. Our results indicate that consumption of α-lactalbumin as a dietary protein has a beneficial effect in not only delaying the initiation of DMBA-induced breast tumors but also in controlling their aggressiveness in comparison to casein fed animal group. Although α-lactalbumin did not prevent the development of mammary tumor in DMBA exposed animals, it supports our hypothesis that it has some protective effect on breast cancer development.
Another interesting point to be noted is that the protection against DMBA-induced breast cancer in rats by α-lactalbumin as noticed in this study is superior to the protection by soy protein reported earlier[1]. For example, the % of rats with tumors at the completion of study is significantly less in the α -lactalbumin group as observed in the present study in comparison to the soy protein group as observed in the earlier study.
We used conventional histological grading to assess and predict the aggressiveness and clinical behavior of mammary gland carcinoma[25]. We graded the tumor on the basis of tubule formation within the neoplasm, nuclear pleomorphism and mitotic count per hpf[1,25]. This study shows that 100% of the breast tumors in the DMBA/α-lactalbumin group was non-aggressive (grade I), whereas the DMBA/casein group had higher percentage of aggressive tumors (20% grade I, 60% grade II and 20% grade III; Table 2). Furthermore, α-lactalbumin had anti-proliferative effect in breast cancer development. Even though the histological grading system applied here was originally developed for human breast carcinoma[25], this system can be successfully applied to rat breast carcinoma[1]. Several studies have demonstrated a similarity in histological characteristics between human and rat breast carcinoma[26,27]. Tumor multiplicity was also lower (1.4 vs. 3.5, p < 0.05) in rats fed α-lactalbumin than that in casein. Ronis et al[24] reported that hydrolytic processing of whey protein is required for this diet to be effective in reducing DMBA-induced mammary tumors.
The proliferative activity of the tumors was assessed by immunohistochemistry with Ki-67 antibody. The degree of Ki-67 positive staining intensity increases with aggressiveness in tumor. Ki-67 staining intensity was significantly reduced in α-lactalbumin group than that in casein group. Positive Ki-67 staining correlates with the degree of differentiation, vascular invasion, lymph node metastases and aggressiveness of the tumors
Besides α-lactalbumin, other active components of whey include lactoferrin, ß-lactoglobulin, serum albumin, glycomacropeptide, lactoperoxidase enzymes, lactose, minerals and immunoglobulins[5]. Whey protein components, ß-lactoglobulin, α-lactalbumin and serum albumin were studied infrequently, but results suggest they have anticancer potential.
The minor component lactoferrin has received the most attention. It has been reported that lactoferrin inhibits intestinal tumors and perhaps tumors at other sites. It acts by induction of apoptosis, inhibition of angiogenesis, modulation of carcinogen metabolizing enzymes and perhaps acting as an iron scavenger. This benefit is attributed to its high content of cystine/cysteine and gamma-glutamylcyst(e)ine dipeptides, which are efficient substrates for the synthesis of glutathione. Glutathione is a ubiquitous cellular antioxidant that directly or through its associated enzymes destroys reactive oxygen species, detoxifies carcinogens, maintains proteins in a reduced state and ensures a competent immune system. Various experiments showed that tumor prevention by dietary whey protein was accompanied by increased glutathione levels in serum and tissues as well as enhanced splenic lymphocyte proliferation, phagocytosis and natural killer, T helper and cytotoxic T cell activity[28].
However, the anti-tumor activity of lactoferrin is controversial. For example, it is known to stimulate the transcription of endothelin-1 (ET-1), a secreted proinvasive polypeptide that acts through a specific receptor, ETAR, leading to secretion of the bioactive ET-1 peptide[29]. ET-1 has been previously shown to promote breast cancer progression, invasion and metastasis[30]. Furthermore, it has been suggested that ET-1, ETAR and ETBR might be used as predictive index of aggressive behavior and invasive/metastatic phenotype because of their over expression in aggressive breast cancers[30,31]. Thus the presence of lactoferrin in dietary whey protein may restrict the beneficial effect of whey in controlling breast cancer. Therefore, it is advisable to use pure α-lactalbumin, another component of whey protein as a therapeutic agent.
Authors’ Contributions: SKD designed, SSR, SM conducted the experiment, BB interpreted histological findings, SSR, SKD wrote the manuscript, SM performed statistical analysis, and all authors participated in the review and approval of the final manuscript.
Acknowledgment
This study was supported by grants from US Army (DAMD17-03-1-0352), Fuji Oil Company, Osaka, Japan and NIH (5U54MD007593). The authors thank Dr. Anuradha Ratna for her help in proofreading of the manuscript.
References
- 1. Mukhopadhyay, S., Ballard, B.R., Mukherjee, S., et al. Beneficial effects of soy protein in the initiation and progression against dimethylbenz [a] anthracene-induced breast tumors in female rats. (2006) Mol Cell Biochem 290(1-2): 169-176.
- 2. Adlercreutz, H., Mousavi, Y., Hockerstedt, K. Diet and breast cancer. (1992) Acta Oncol 31(2): 175-181.
- 3. Tsuda, H., Sekine, K., Ushida, Y., et al. Milk and dairy products in cancer prevention: focus on bovine lactoferrin. (2000) Mutat Res 462(2-3): 227–233.
- 4. Bounous, G., Batist, G., Gold, P. Whey protein in cancer prevention. (1991) Cancer Lett 57(2): 91–94.
- 5. Marshall, K. Therapeutic applications of whey protein. (2004) Altern Med Rev 9(2): 136–156.
- 6. Bounous, G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. (2000) Anticancer Res 20(6C): 4785-4792.
- 7. Walzem, R.L., Dillard, C.J., German, J.B. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. (2002) Crit Rev Food Sci Nutr 42(4): 353–375.
- 8. Daenzer, M., Petzke, K.J., Bequette, B.J., et al. Whole body nitrogen and splanchnic amino acid metabolism differ in rats fed mixed diets containing casein or its corresponding amino acid mixture. (2001) J Nutri 131(7): 1965–1972.
- 9. Quig, D. Cysteine metabolism and metal toxicity. (1998) Altern Med Rev 3(4): 262–270.
- 10. Hakkak, R.S., Korourian, S., Ronis, M.J., et al., Dietary whey protein protects against azoxymethane-induced colon tumors in male rats. (2001) Cancer Epidemiol Biomarkers Prev 10(5): 555–558.
- 11. Hakkak, R.S., Korourian, S., Shelnutt, S.R., et al. Diets containing whey proteins or soy protein isolate protect against 7, 12-Dimethylben (a) anthracene-induced mammary tumors in female rats. (2000) Cancer Epidemiol Biomarkers Prev 9(1): 113–117.
- 12. Van Dael, P., Kastenmayer, P., Clough, J., et al. Substitution of casein by ß-casein or of whey protein isolate by α-lactalbumin does not affect mineral balance in growing rats. (2005) J Nutri 135(6): 1438-1443.
- 13. Permyakov, S.E., Pershikova, I.V., Zhadan, A.P., et al. Conversion of human α-lactalbumin to apo-like state in the complexes with basic poly-amino acids: towards understanding of the molecular mechanism of antitumor action of HAMLET. (2005) J Proteome Res 4(2): 564–569.
- 14. Hakansson, A., Zhivotovsky, B., Orreniuset, S., et al. Apoptosis induced by a human-milk protein. (1995) Proc Natl Acad Sci USA 92(17): 8064–8068.
- 15. Pellegrini, U.T., Bramaz, N., Hunziker, P., et al. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. (1999) Biochim Biophys Acta 1426(3): 439–449.
- 16. Permyakov, S.E., Pershikova, I.V., Khokhlova, T.I., et al. No need to be HAMLET or BAMLET to interact with histones: binding of monomeric α-lactalbumin to histones and basic poly-amino acids. (2004) Biochemistry 43(19): 5575–5582.
- 17. Kohler, C., Håkansson, A., Svanborg, C., et al. Protease activation in apoptosis induced by MAL. (1999) Exp Cell Res 249(2): 260–268.
- 18. Kohler, C., Gogvadze, V., Håkansson, A., et al. A folding variant of human α-lactalbumin induces mitochondrial permeability transition in isolated mitochondria. (2001) Eur J Biochem 268(1): 186-191.
- 19. Casbarra, A., Birolo, L., Infusini, G., et al. Conformational analysis of HAMLET, the folding variant of human α-lactalbumin associated with apoptosis. (2004) Protein Sci 13(5): 1322–1330.
- 20. Rowlands, J.C., He, L., Hakkak, R., et al. Soy and whey proteins downregulate DMBA-induced liver and mammary gland CYP1 expression in female rats. (2001) J Nutri 131(12): 3281-3287.
- 21. Rowlands, J.C., Hakkak, R., Ronis, M.J., et al. Altered mammary gland differentiation and progesterone receptor expression in rats fed soy and whey proteins. (2002) Toxicol Sci 70(1): 40-45.
- 22. Salvesen, H.B., Iversen, O.E., Akslen, L.A. Prognostic significance of Angiogenesis and Ki-67, p53 and p21 expression: a population-based endometrial carcinoma study. (1999) J Clin Oncol 17(5): 1382-1390.
- 23. Lee, H.P., Gourley, L., Duffy, S.W., et al. Dietary effects on breast-cancer-risk in Singapore. (1991) Lancet 337(8751): 1197-1200.
- 24. Ronis, M.J., Hakkak, R., Korourian, S., et al. Whey protein hydrolysate but not whole whey protein protects against 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. (2015) Nutr Cancer 67(6): 949-953.
- 25. Elsion, C.W., Ellis, I.O. Pathological prognostic factors in breast cancer: The value of histological grade in breast cancer: experience from a large study with long-term follow up. (1991) Histopathology 19(5): 403-410.
- 26. Russo, J., Gusterson, B.A., Rogers, A.E., et al. Comparative study of human and rat mammary tumorigenesis. (1990) Lab Invest 62(3): 244-278.
- 27. Young, S., Hallowes, R.C. Tumors of the mammary gland. In: Turusov, V.S., ed. Pathology of Tumors in Laboratory Animals. (1973) IARC Scientific Publications, Lyon, France 1(5): 31-73.
- 28. Parodi, P.W. A role for milk proteins and their peptides in cancer prevention. (2007) Curr Pharm Des 13(8): 813-828.
- 29. Ha, N.H., Nair, V.S., Reddy, D.N., et al. Lactoferrin-endothelin-1 axis contributes to the development and invasiveness of triple-negative breast cancer phenotypes. (2011) Cancer Res 71(23): 7259-7269.
- 30. Wulfing, P., Diallo, R., Kersting, C., et al. Expression of endothelin-1, endothelin-A, and endothelin-B receptors in human breast cancer and correlation with long-term follow-up. (2003) Clin Cancer Res 9(11): 4125-4131.
- 31. Bendinell, P., Maroni, P., Matteucci, E., et al. Microenvironmental stimuli affect endotheil-1 signaling responsible for invasiveness and osteomimicry of bone metastasis from breast cancer. (2014) Biochim Biophys Acta 1843(4): 815-826.