Role of the Biofield Energy Treated Test Formulation on Different Vital Organ Specific Biomarkers
William Dean Plikerd1, Mahendra Kumar Trivedi1, Alice Branton1, Dahryn Trivedi1, Gopal Nayak1, Mayank Gangwar2, and Snehasis Jana2*
Affiliation
1Trivedi Global, Inc., Henderson, Nevada, USA
2Trivedi Science Research Laboratory Pvt. Ltd., Thane-West, Maharashtra, India
Corresponding Author
Snehasis Jana, Trivedi Science Research Laboratory Pvt. Ltd., Thane-West, Maharashtra, India, E-mail: publication@trivedieffect.com
Citation
Plikerd, WD., et al. Role of the Biofield Energy Treated Test Formulation on Different Vital Organ Specific Biomarkers. (2019) Lett Health Biol Sci 4(1): 16-25.
Copy rights
© 2019 Plikerd, WD. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
The Trivedi Effect®; Biofield energy treatment; Organ health; Multiple organ failure; Bone health
Abstract
The present study was undertaken to evaluate the impact of the Biofield Energy Treated test formulation using cell lines related with vital organs functioning. In vitro cells based assay were performed to study the effects on the bones, heart, liver, lungs, and brain cells. The test formulation and the cell media was divided into two parts; one part was untreated (UT) and other part received the Biofield Energy Treatment remotely by a renowned Biofield Energy Healer, William Dean Plikerd, USA and labeled as the Biofield Energy Treated (BT) test formulation/media. The test formulation was tested against various activities using cell line assay in their specific medium (Med). The test formulation was tested for cell viability, and the results showed that the test formulation at tested concentrations was found safe and non-toxic. Cytoprotective action of the test formulation showed a significant maximum restoration of cell viability by 133.4% (at 1 µg/mL), 65.7% (at 10 µg/mL), and 86.8% (at 10 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, BT-Med + BT-TI groups respectively, as compared to the untreated test group in human cardiac fibroblasts cells (HCF) cells, while improved restoration of cell viability by 97.4% (at 1 µg/mL), 85.7% (at 10 µg/mL), and 81.9% (at 10 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, BT-Med + BT-TI groups respectively, as compared to the untreated test group in HepG2 cells. Cellular restoration in A549 cells was improved by 174.2%, 472.6%, 118%, and 279.2% at 0.1, 1, 10, and 25.5 µg/mL respectively, in the BT-Med + UT-TI group, while 1514%, 1654.8%, 449.8%, and 540.4% improved cellular restoration was reported at 0.1, 1, 10, and 25.5 µg/mL respectively, at BT-Med + BT-TI groups as compared to the untreated test group. ALP activity in MG-63 cells was significantly increased by 93.4% at 50 µg/mL in the UT-Med + BT-TI group, while in Ishikawa cells showed maximum increased ALP activity by 107.4% at 50 µg/mL in the BT-Med + UT-TI group as compared to the untreated group. The maximum percent cellular protection of HCF (heart) cells (decreased of LDH activity) was significantly increased by 35.9% at 10 µg/mL in the UT-Med + BT-TI group, while BT-Med + UT-TI group showed increased protection by 69.3% at 1 µg/mL, and improved cellular protection by 119.1% and 44% at 1 and 10 µg/mL respectively, in the BT-Med + BT-TI group as compared to the untreated test group. Alanine amino transferase (ALT) activity was reported in terms of percent cellular protection of HepG2 (liver) cells. Results showed improved HepG2 cells protection (represents decreased ALT activity) by 23.8% (at 10 µg/mL), 98.1% (at 25.5 µg/mL), and 97.6% (at 25 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, BT-Med + BT-TI groups respectively, as compared to the untreated test group. Percentage cellular protection of A549 (lungs) cells (represents increased of SOD activity) was increased by 73.5%, 109.8%, and 97.7% at 0.1 µg/mL in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to untreated group. Serotonin level was significantly increased 68% (at 25 µg/mL), 75.7% (at 0.1 µg/mL), and 57.2% (at 25 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively compared to untreated test group in human neuroblastoma cells (SH-SY5Y). However, the relative quantification (RQ) of vitamin D receptor (VDR) was significantly increased by 16.4% (at 10 µg/mL), 182% (at 50 µg/mL), and 137.1% (at 50 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups, respectively as compared to the untreated in MG-63 cells. In conclusion, Biofield Energy treated test formulation (The Trivedi Effect®) would be significantly useful for multiple organs health that can be used against coronary artery disease, arrhythmias, congenital heart disease, cardiomyopathy, cirrhosis, liver cancer, hemochromatosis, asthma, chronic bronchitis, cystic fibrosis, osteoporosis, etc.
Introduction
In modern societies, healthy ageing and wellbeing are one of the foremost criteria common goals in modern societies. Thus, most of the research has been focused on health life and organ health, which directly affects the aging and lifespan. Biological processes are contributing a huge impact on human health, but the majority of the health issues are related with the multiple organ failure, which modulate ageing along with the risk of age-related frailty, disability, and associated diseases[1]. Thus, some standard cellular biomarkers of healthy ageing correlating with the organ health are the current utility as surrogate endpoints of research. Various pre-clinical and clinical trials have been reported, which focused to develop some formulation that works to improve the overall health[2]. However, there is no such novel herbal-based test formulation was designed that can improve the overall organ health using cell based standard assays. There is currently no universally accepted test formulation, which improve the organ health biomarkers. With this respect, the novel test formulation was designed on the basis of best scientific literature, which is the combination of herbal products viz. panax ginseng extract and beta carotene, minerals viz. calcium chloride, magnesium gluconate, zinc chloride, sodium selenate, ferrous sulfate, and vitamins viz. vitamin B12, vitamin D3, ascorbic acid, and vitamin B6. This formulation is designed for overall functioning of the organs that can results in improved overall health conditions against many pathological conditions such as lung disorder, liver disorder, breast cancer, liver cancer, aging, muscle damage, and overall health. Minerals and vitamins present in the test formulation provide significant functional support to all the vital organs[3-5]. In addition, panax ginseng is one of the best reported medicinal plants that improve mental, physical abilities, cognitive health, and is potent immunomodulator[6,7]. The test formulation was tested against many cell lines and was evaluated for biological activities such as bone health parameters using MG-63 cells, lung health parameter using A549 cells, liver health parameter using HepG2 cells, heart health parameter using Human Cardiac fibroblasts, and neuronal health parameter using SH-SY5Y cells[8-17].
The test formulation was first treated with the Biofield Energy through a renowned Biofield Energy Healer, which was tested for various test parameters. Biofield Energy Healing Treatment, one of the emerging Complementary and Alternative Medicine (CAM) approaches, which has been reported with significant results[18-20]. The recent scientific reports stated that human Biofield Energy is capable of significantly suppression of mouse lung carcinoma growth along with significant immune function and anti-inflammatory activity using various molecular biomarkers[21]. Thus, Biofield Energy Healing therapies have gained popularity because of improved immunological response[22]. Besides, CAM therapies have been recommended by The National Center for Complementary/Alternative Medicine (NCCAM) and there therapies exist in various therapies such as external qigong, Johrei, Reiki, therapeutic touch, yoga, Qi Gong, polarity therapy, Tai Chi, pranic healing, deep breathing, chiropractic/osteopathic manipulation, guided imagery, meditation, massage, homeopathy, hypnotherapy, progressive relaxation, acupressure, acupuncture, special diets, relaxation techniques, Rolfing structural integration, healing touch, movement therapy, pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems[23,24]. The Trivedi Effect®-Consciousness Energy Healing therapies have been widely accepted and popular worldwide healing approach with significant results in many scientific field. The Trivedi Effect® has been reported with significant results in the metal physicochemical properties[25,26], agriculture science[27], microbiology[28,29], biotechnology[30,31], improved bioavailability of many compounds[32,33], skin health[34,35], nutraceuticals[36], cancer science research[37], improved bone health[38-40], human health and wellness. Due to the continued clinical and preclinical applications of Biofield Energy Healing Treatments, the test formulation was studied for impact of the Biofield Energy Healing Treated test formulation on the function of vital organs such as bones, heart, liver, lungs, and brain specific biomarkers in different cell-lines.
Materials and Methods
Chemicals and reagents
All the chemicals used in the experiment were procured from standard company. Panax ginseng extract obtained from panacea Phytoextracts, India. Sodium selenate and ascorbic acid were obtained from Alfa Aesar, India. Silymarin and curcumin were obtained from Sanat Chemicals, India and quercetin was purchased from Clearsynth, India. Ferrous sulfate, vitamin B6, vitamin D3, vitamin B12, calcium chloride, naringenin, trimetazidine (TMZ), 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT), and ethylenediaminetetraacetic acid (EDTA) were procured from Sigma Chemical Co. (St. Louis, MO). Zinc chloride, magnesium gluconate, β-carotene, and calcitriol were purchased from TCI chemicals, Japan. Reverse Transcription Kit, RNeasy Mini Kit, and Syber Green PCR kits were procured from Qiagen, India. All the other chemicals used in this experiment were analytical grade procured from India.
Biofield energy healing treatment
Biofield Energy Healing Treatment was performed on the test formulation and the specific medium used for cell lines. The test formulation was the combination of eleven ingredients such as panax ginseng extract, β-carotene, zinc chloride, calcium chloride, magnesium gluconate, sodium selenate, ferrous sulfate, ascorbic acid, vitamin B12, vitamin D3, and vitamin B6. The test formulation and the medium were divided into two parts, one portion was considered as the untreated group, where no Biofield Energy Treatment was provided (UT-TI and UT-Med). Further, the untreated group was treated with a “sham” healer for comparison purposes, who did not have any knowledge about the Biofield Energy Healing Treatment. Another test formulation portion and the medium received the Biofield Energy Treatment (The Trivedi Effect®) remotely by William Dean Plikerd, under standard laboratory conditions for ~3 minutes through healer’s unique Biofield Energy Transmission process and were referred as the Biofield Energy Treated formulation (BT-TI) and Biofield Energy Treated medium (BT-Med). The Biofield Energy Healer was located in the USA, however the test formulation constituents were located in the research laboratory of Dabur Research Foundation, New Delhi, India. Biofield Energy Healer in this experiment did not visit the laboratory, nor had any contact with the test sample and the medium. After that, the Biofield Energy Treated and untreated test items were kept in similar sealed conditions and used for the study as per the study plan.
Cell viability testing using MTT assay
All the experimental cells used in this study were counted for cell viability using hemocytometer in 96-well plates at the specific density as mentioned in the Table 1. The cells were then incubated overnight under standard growth conditions to allow cell recovery and exponential growth. Following overnight incubation, cells were treated with different concentrations of test formulations (BT/UT). After respective treatments, the cells were incubated in a CO2 incubator at 37°C, 5% CO2, and 95% humidity. After incubation, the plates were taken out and 20 µL of 5 mg/mL of MTT 3-(4,5-dimethythiazol-2-yl)-2, 5-diphenyl tetrazolium bromide solution was added to all the wells followed by additional incubation for 3 hours at 37°C. The supernatant was aspirated and 150 µL of DMSO was added to each well to dissolve formazan crystals. The absorbance of each well was read at 540 nm using Synergy HT microplate reader. The percentage cytotoxicity at each tested concentration was calculated using Equation 1:
% Cytotoxicity = [(R-X)/R] *100............ (1)
Where, X = Absorbance of treated cells; R = Absorbance of untreated cells
The concentrations exhibiting percentage cytotoxicity <30% was considered as non-cytotoxic[41].
Table 1: Information related to six cell lines with their plating density and time-point.
|
S. No. |
Cell Line |
Plating |
Time Point |
|
1 |
MG-63 (Bone) |
3x104 cells/ well, 96-well plate |
5 days |
|
2 |
Ishikawa (Uterus) |
3x104 cells/ well, 96-well plate |
5 days |
|
3 |
A549 (Lung) |
10x104 cells/ well, 96-well plate |
24 hours |
|
4 |
HepG2 (Liver) |
1x104 cells/ well, 96-well plate |
24 hours |
|
5 |
Human Cardiac fibroblasts (Heart) |
1x104 cells/ well, 96-well plate |
24 hours |
|
6 |
SH-SY5Y (Neuronal cell) |
10x104 cells/ well, 96-well plate |
24 hours |
Cytoprotective action of the test formulation
Cytoprotective effect of the test formulation in selected cells such as human cardiac fibroblasts-HCF; human hepatoma cells-HepG2; and adenocarcinomic human alveolar basal epithelial cells-A549 were counted and plated in suitable medium followed by overnight incubation. Further, the cells were then treated with the test items/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, the oxidative stress using 10 mM t-BHP for 3.5 hours was given to the cells. The cells treated with 10 mM of t-BHP alone served as negative control. After 3.5 hours of incubation with t-BHP the above plates were taken out and cell viability was determined by MTT assay. The percentage protection corresponding to each treatment was calculated using equation 2:
% Protection = [(Absorbancesample-Absorbancet-BHP)]*100/ [Absorbanceuntreated-Absorbancet_BHP]............... (2)
Estimation of alkaline phosphatase (ALP) activity
For the estimation of ALP, two cells such as human bone osteosarcoma cells-MG-63 and human endometrial adenocarcinoma cells-Ishikawa were counted using a hemocytometer and plated in 24-well plates at the density corresponding to 1 X 104 cells/well in phenol-free DMEM supplemented with 10% CD-FBS. After the respective treatments, the cells in the above plate were incubated for 48 hours in CO2 incubator at 37°C, 5% CO2, and 95% humidity. After 48 hours of incubation, the plates were taken out and processed for the measurement of ALP enzyme activity. The cells were washed with 1 X PBS and lysed by freeze-thaw method i.e., incubation at -80°C for 20 minutes followed by incubation at 37°C for 10 minutes. To the lysed cells, 50 µL of substrate solution i.e. 5 mM of p-nitrophenyl phosphate (pNPP) in 1M diethanolamine and 0.24 mM magnesium chloride (MgCl2) solution (pH 10.4) was added to all the wells followed by incubation for 1 hour at 37°C. The absorbance of the above solution was read at 405 nm using Synergy HT microplate reader (Biotek, USA). The absorbance values obtained were normalized with substrate blank (pNPP solution alone) absorbance values. The percentage increase in ALP enzyme activity with respect to the untreated cells (baseline group) was calculated using Equation 3:
% Increase in ALP = {(X-R)/R}*100----------------------- (3)
Where,
X = Absorbance of cells corresponding to positive control and test groups
R = Absorbance of cells corresponding to baseline group (untreated cells)
Estimation of lactate dehydrogenase (LDH) in human cardiac fibroblasts (HCF) cells
HCF cells were used for the estimation of LDH activity. The cells were counted and plated at the density of 0.25 X 106 cells/ well in 24-well plates in cardiac fibroblast specific mediumfollowed by overnight incubation. The cells were then treated with the test formulation combinations/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, oxidative stress was given to the cells using 10 mM t-BHP for 3.5 hours. The untreated cells were served as control group, which did not receive any treatment and were maintained in cell growth medium only. Cells treated with 10 mM of t-BHP alone served as the negative control. After 3.5 hours of incubation with t-BHP, the above plates were taken out and LDH activity was determined using LDH activity kit as per manufacturer’s instructions. The percent increase in LDH activity was calculated using Equation 4.
% Increase = [(LDH activitysample-LDH activityt-BHP)]*100/ [LDH activityuntreated-LDH activityt_BHP].............. (4)
Estimation of ALT in liver cells (HepG2)
The human hepatoma cells (HepG2) were used for the estimation of ALT activity. The cells were counted and plated at the density of 5 X 104 cells/well in 48-well plates in DMEM media followed by overnight incubation. The cells were then treated with the test formulation/positive control at the non-cytotoxic concentrations for 24 hours. After 24 hours, oxidative stress was given to the cells using 400 µM t-BHP for 3.5 hours. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. Cells treated with 400 µM of t-BHP alone served as negative control. After 3.5 hours of incubation with t-BHP, the above plates were taken out and ALT activity was determined using ALT activity kit as per manufacturer’s instructions. The percent increase in ALT activity was calculated using Equation 5.
% Increase = [(ALT activitysample-ALT activityt-BHP)]*100/ [ALT activityuntreated-ALT activityt_BHP]............. (5)
Estimation of superoxide dismutase (SOD) in lung (A549) cells
The adenocarcinomic human alveolar basal epithelial cells (A549) were used for the estimation of SOD activity. The A549 cells were counted and plated at the density of 1 X 104 cells/well in 24-well plates in DMEM followed by overnight incubation. The cells were then treated with the test formulation/positive control at the non-cytotoxic concentrations along with 100 µM t-BHP to induce oxidative stress. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. Cells treated with 100 µM of t-BHP alone served as negative control. After 24 hours of incubation with t-BHP the above plates were taken out and SOD activity was determined using SOD activity kit as per manufacturer’s instructions. The percent increase in SOD activity was calculated using equation 6:
% Increase in SOD activity = ((X-R)/R)*100................ (6)
Where,
X = SOD activity corresponding to test item or positive control
R = SOD activity corresponding to Control group.
Estimation of serotonin in neuronal cells (SH-SY5Y)
The human neuroblastoma (SH-SY5Y) cells were used for the estimation of serotonin level. Te cells were counted and plated at the density of 10 X 104 cells/well in 96-well plates followed by overnight incubation. The cells were then treated with the test formulation/positive control at the non-cytotoxic concentrations. The untreated cells served as control that did not receive any treatment and were maintained in cell growth medium only. The treated cells were incubated for 24 hours. Serotonin release was determined by ELISA as per manufacturer’s protocol. The percent increase in serotonin levels was calculated using equation 7-
[(X-R)/R]*100................ (7)
Where,
X = Serotonin levels corresponding to test item or positive control,
R = Serotonin levels corresponding to control group.
Effect of test formulation on vitamin D receptor (VDR) in bone (MG-63) cells
The effect of test formulation on vitamin D receptor (VDR) activity in bone (MG-63) cells were counted using the hemocytometer at density 2 X 105 cells/well in 6-well plates followed by overnight incubation. The cells were then sera starved for 24 hours and treated with the test formulation/positive control at the non-cytotoxic concentrations, while control group did not receive any treatment, which were maintained in cell growth medium only. The treated cells were incubated for 24 hours and VDR expression was determined by qPCR using VDR specific primers. Cells were harvested by scrapping and washed with PBS. Cell pellets obtained were analyzed for VDR gene expression using human VDR specific primers: Forward: 5’-GCTGACCTGGTCAGTTACAGCA-3’, Reverse: 5’-CACGTCACTGACGCGGTACTT-3’.VDR gene expression was normalized using House-keeping (HK) reference. Relative quantification (RQ) of VDR gene in Biofield Energy Treated cells was calculated with respect to the untreated cells using equation 8:
RQ = 2-N................ (8)
Where, N is the relative Threshold Cycle (CT) value of treated sample with respect to the untreated sample.
Statistical analysis
All the experimental values were presented as mean ± SD (standard deviation) of three independent experiments. The statistical analysis was performed using SigmaPlot statistical software (v11.0). For two group comparison, student’s t-test was used. For multiple group comparison, one-way analysis of variance (ANOVA) was used followed by post-hoc analysis by Dunnett’s test. Statistically significant values were set at the level of p ≤ 0.05.
Results and Discussion
Cell viability using MTT assay
MTT assay for cell viability testing was used in each cell lines for testing the safe concentrations. The test concentrations of the formulation were found safe on the basis of percentage of cell viability. The test criteria for non-cytotoxic test formulation concentration and the positive controls were found to be less than 30% cytotoxicity or greater than 70% cell viability. All the results were considered and represented as safe and non-cytotoxic concentrations. Overall, the experimental data suggested that the overall percent cell viability in different cell-lines viz. MG-63, Ishikawa, A549, HepG2, HCF, and SH-SY5Y were found safe, which were tested for other activities.
Evaluation of cytoprotective effect of the test formulation
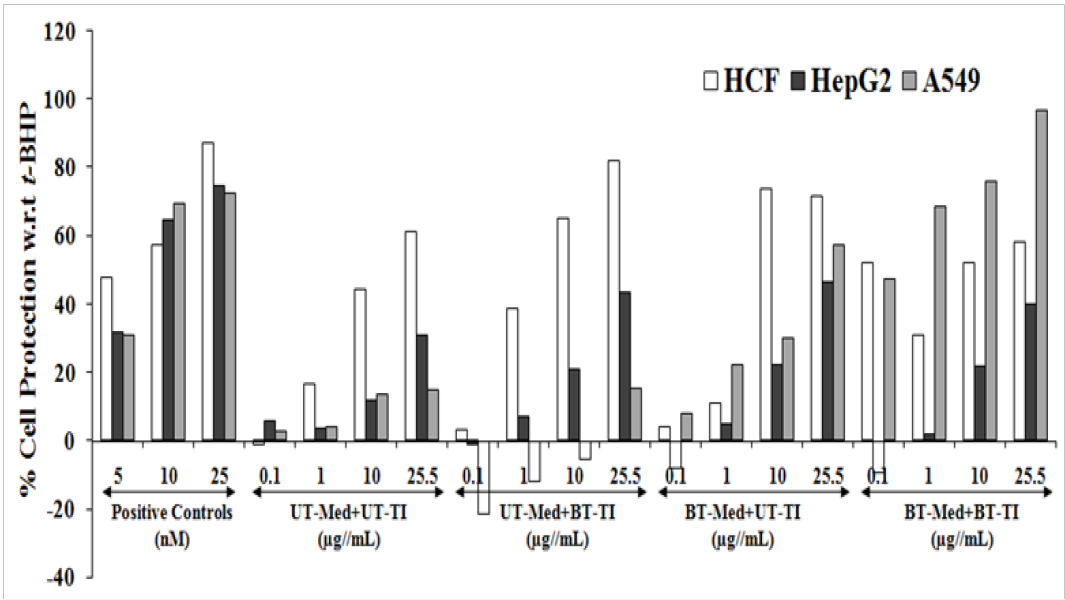
The test formulation was screened of cytoprotective activity against three cell lines viz. HCF, HepG2, and A549 cells, while the data was presented in terms of percentage cell protection against t-BHP induced cell damage (Figure 1). Trimetazidine (TMZ) was used as a positive control group in human cardiac fibroblasts cells (HCF) for cytoprotective effect which showed significant restoration of cell viability by 48%, 57.2%, and 87.2% at 5, 10, and 25 µg/mL, respectively as compared to the t-BHP induced group. Besides, the restoration of cell viability among the tested groups by the test formulation was reported as 133.4%, 45.9%, and 34% at 1, 10, and 25 µg/mL respectively, in the UT-Med + BT-TI group, while 65.7% and 16.9% improved cellular restoration at 10 and 25 µg/mL respectively, in the BT-Med + UT-TI, and 86.8% and 17.2% improved cellular restoration at 10 and 25 µg/mL respectively, in the BT-Med + BT-TI group as compared to the untreated test group (UT-Med + UT-TI group). Similarly, silymarin was used as positive control in HepG2 cells, which resulted in significant cellular restoration by 31.6%, 64.6%, and 74.6% at 5, 10 and 25 µg/mL respectively, as compared to the t-BHP induced group. Besides, the test formulation showed maximum restoration of cell viability by 97.4%, 73%, and 39.4% at 1, 10, and 25 µg/mL respectively, in the UT-Med + BT-TI group, while 43.4%, 85.7%, and 49.9% improved cellular restoration at 1, 10, and 25 µg/mL respectively, in the BT-Med + UT-TI, and 81.9% and 28.6% improved cellular restoration at 10 and 25 µg/mL respectively, in the BT-Med + BT-TI group as compared to the untreated test group (UT-Med + UT-TI group). In addition, quercetin was used as positive control in adenocarcinomic human alveolar basal epithelial cells (A549) resulted, restoration of cell viability by 30.8%, 69.3%, and 72.2% at 5, 10 and 25 µg/mL, respectively compared to the t-BHP induced group. Besides, the test formulation showed maximum restoration of cell viability by 174.2%, 472.6%, 118%, and 279.2% at 0.1, 1, 10, and 25.5 µg/mL respectively, in the BT-Med + UT-TI group. Similarly, 1514%, 1654.8%, 449.8%, and 540.4% improved cellular restoration was reported at 0.1, 1, 10, and 25.5 µg/mL respectively, at BT-Med + BT-TI groups as compared to the UT-Med + UT-TI group. This shows significant cytoprotective activity after oxidative stress using tert-butyl hydroperoxide (t-BHP). This method has been considered as the gold standard for testing the cytoprotective action by stimulation in cell based assay[41,42]. This activity would reflects that the test formulation could be one of the best tool to protect the cell against injuries due to free radicals and many other factors such as oxidative stress[43,44] and would help to protect against many immune related disorders such as cardiovascular diseases, aging, cancer, diabetes, and many more[45-47]. Overall, it can be assumed that significant improved cellular restoration was reported due to Biofield Energy Treatment (The Trivedi Effect®) and it significantly protects the t-BHP induced oxidative stress against the HCF, HepG2, and A549 cells with respect to the cardiotoxicity, hepatotoxicity, and lung cell toxicity. Therefore, the Biofield Energy Healing Treatment could be used against many pathological etiologies such as cardiovascular, liver, and lung diseases.
Figure 1: Cytoprotective action of the test formulation in human cardiac fibroblasts cells (HCF), human hepatoma cells (HepG2), and adenocarcinomic human alveolar basal epithelial cells (A549) against tert-butyl hydroperoxide (t-BHP) induced damage. Trimetazidine (µM), silymarin (µg/mL), and quercetin (µM) were used as positive control in HCF, HepG2, and A549 cells, respectively. UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation of Alkaline Phosphatase (ALP) Activity
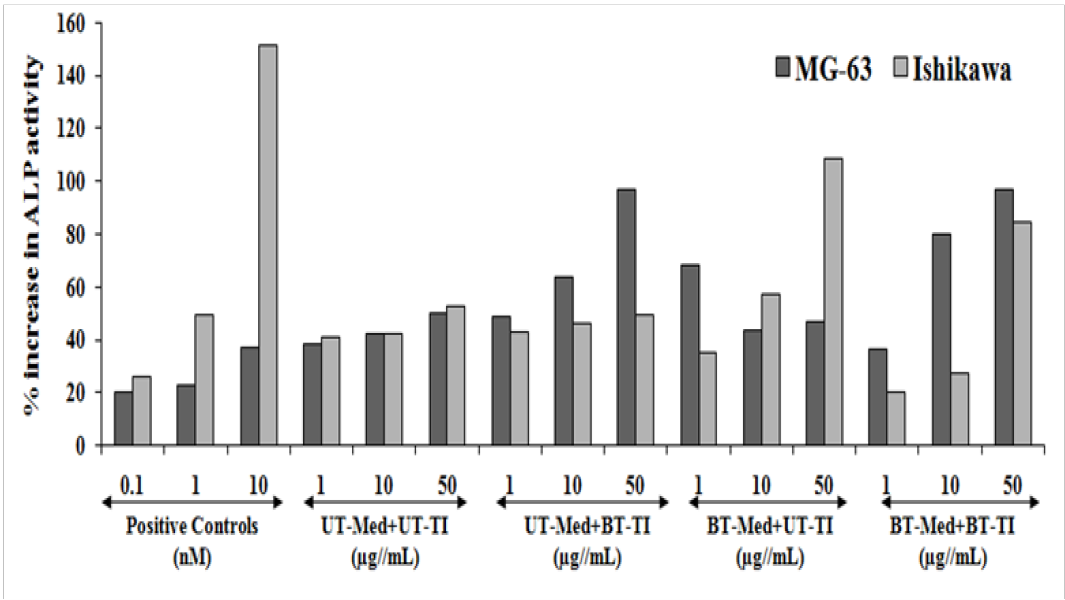
ALP activity was evaluated against two cell lines, MG-63 and Ishikawa cells after treatment with the test formulation. Naringenin (nM) was used as positive control in Ishikawa cells, and the results suggested significant increased ALP level by 25.9%, 49.2%, and 151.9% at 0.1, 1, and 10 nM respectively as shown in the Figure 2. However, the experimental test groups showed maximum increased ALP activity by 6.1% and 10.3% at 1 and 10 µg/mL respectively, in the UT-Med + BT-TI, while 35.9% and 107.4% increased ALP activity at 10 and 50 µg/mL respectively, in the BT-Med + UT-TI group, and 60.9% improved ALP level was found at 50 µg/mL in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group in Ishikawa cells. Similarly, calcitriol was used as positive control for MG-63 cells, and the data showed significant improved level of ALP by 20%, 22.7%, and 36.8% at 0.1, 1, and 10 nM, respectively. In the experimental tested group of MG-63 cells, the ALP percent was significantly increased by 26.7%, 51.4%, and 93.4% at 1, 10, and 50 µg/mL, respectively in the UT-Med + BT-TI group as compared to the UT-Med + UT-TI group. Similarly, ALP percent was significantly increased by 77.9% and 3% at 1 and 10 µg/mL, respectively in the BT-Med + UT-TI group as compared to the UT-Med + UT-TI group. However, ALP percent was significantly increased by 90.3% and 93.2% at 10 and 50 µg/mL, respectively in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group in the MG-63 cells. Overall, it can be concluded significant improved ALP level after treatment with the Biofield Energy Healing Treatment. ALP is one of the important bone health biomarker responsible for controlling various bone disorders[48,49] like low bone density and osteoporosis, osteogenesis imperfect and Paget’s disease, which makes bones brittle. Thus, Biofield Energy Treated Test formulation would be highly recommended option in bone disorders without any adverse effects in comparison with the synthetic drugs.
Figure 2: Alkaline phosphatase (ALP) activity in human bone osteosarcoma cells (MG-63) and human endometrial adenocarcinoma cells (Ishikawa) after treatment of the test formulation. Calcitriol and naringenin were used as positive control in MG-63 and Ishikawa cells, respectively. UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Lactate dehydrogenase (LDH) activity in human cardiac fibroblasts (HCF)
LDH activity was estimated in HCF cells, as LDH is an enzyme found in all the living cells and found to be responsible for anaerobic cellular respiration. The data was presented as increased HCF cells cellular protection, which represents decreased LDH activity in various groups. The effect of test formulation in different groups with respect to the percent protection of HCF cells in terms of decreased level of lactate dehydrogenase (LDH) activity is presented in the Figure 3. The positive control, trimetazidine (TMZ) showed 63.1%, 92.3%, and 115.2% increased cellular protection of HCF cells (decreased of LDH activity) at 10, 50, and 100 µM concentration as compared to the t-BHP group. The test formulation showed maximum percent protection of HCF cells (decreased of LDH activity), which was significantly increased by 35.9% and 28.4% at 10 and 25.5 µg/mL concentrations respectively, in the UT-Med + BT-TI group, while 69.3% and 52.3% improved cellular protection (decreased of LDH activity) at 1 and 10 µg/mL respectively, in the BT-Med + UT-TI group, and 119.1%, 44%, and 11.9% improved cellular protection (decreased of LDH activity) at 1, 10, and 25.5 µg/mL respectively, in BT-Med + BT-TI group as compared to the UT-Med + UT-TI group. Thus, the results suggested that significant reduced level of LDH activity after treatment with the test formulation. LDH is extensively expressed in most of the body tissues, such as blood cells, skeletal muscle, and heart muscle and play a vital role in tissue injury, necrosis, hypoxia, hemolysis, or malignancies. Besides, LDH is the best biomarker for heart disease or tissue injuries. LDH activity can be best depicted using HCF cells, as these cells play a central role in the extracellular matrix maintenance of the normal heart along with synthesis of growth factors and cytokines[50-52]. In conclusion, LDH activity using HCF cells was significantly reduction after Biofield Energy Treatment that could be useful against various pathological conditions such as tissue injury, necrosis, hypoxia, hemolysis or malignancies.
Figure 3: The effect of the test formulation on the increased percent protection of HCF cells, which represents decreased lactate dehydrogenase (LDH) activity against tert-butyl hydroperoxide (t-BHP) induced damage. TMZ: Trimetazidine; UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation of alanine amino transferase (ALT) activity in HepG2 cells
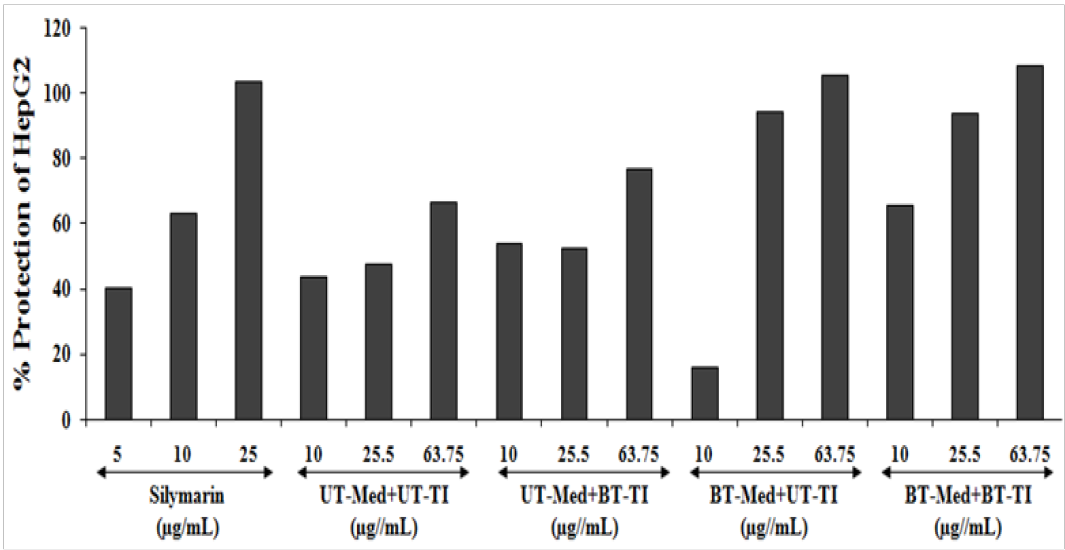
ALT activity was estimated with the help of HepG2 cell and the results are presented in terms of increased percentage cellular protection (which represents decreased ALT activity) in the Figure 4. The positive control, silymarin was in HepG2 cells for ALT activity and the data suggested increased percentage cellular protection of HepG2 cell (decreased ALT activity) by 40.2%, 63.4%, and 103.7% at 5, 10, and 25µg/mL concentrations, respectively. Similarly, the test formulation groups showed improved cellular protection of HepG2 cells (i.e. decreased of ALT activity) by 23.8%, 10.8%, and 14.7% at 10, 25.5, 63.75 µg/mL respectively, in the UT-Med + BT-TI group, while increased cellular protection of HepG2 cells (decreased of ALT activity) by 98.1% and 58% at 25.5 and 63.75 µg/mL respectively, in the BT-Med + UT-TI group, and increased cellular protection of HepG2 cells (decreased of ALT activity) by 50.4%, 97.6%, and 62.2% at 10, 25, and 63.75 µg/mL respectively, in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group (Figure 4). ALT is one of the important liver health enzymes along with kidney, heart, and muscles. Up and down regulation of this enzyme may results in hepatocellular injury and death[53]. Hepatic cellular damage has been linked with high level of ALT, which affects the cell viability and damage to the cells[54]. Biofield Energy Treatment significantly improved the cellular protection with reduced ALT enzyme, which suggests its application in the liver cancer, liver cirrhosis, hepatomegaly, liver failure, and hepatitis.
Figure 4: The increased percentage protection of liver cells (HepG2) that represents decreased (ALT) Alanine amino transaminase activity under the stimulation of tert-butyl hydroperoxide (t-BHP). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Superoxide dismutase (SOD) activity in adenocarcinomic human alveolar basal epithelial cells (A549)
SOD activity was estimated using A549 cells and improved activity represents the increased cellular protection (Figure 5). The positive control, quercetin showed improved percentage increase in the SOD activity with respect to the t-BHP by 74%, 89.8%, and 129.9% at 10, 25, and 50 µg/mL concentration respectively. However, the percent protection of A549 (lungs) cells (increased of SOD activity) was significantly increased by 73.5% and 4.4% at 0.1and 63.75 µg/mL respectively, in the UT-Med + BT-TI group, while increased SOD activity by 109.8%, 19.5%, and 21% at 0.1, 25, and 63.75 µg/mL respectively, in the BT-Med + UT-TI group, and increased SOD activity by 97.7%, 3.2%, and 16.4% at 0.1, 25, and 63.75 µg/mL respectively, in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group (Figure 5). SOD is one the best antioxidant defense mechanism of the body, which prevent the cellular damage against various types of stress and free radicals, which results in cell death[55]. The present experimental data revealed that the Biofield Energy Treatment has significantly improved the SOD antioxidant defense activity, which could protect from many respiratory diseases such as pneumonia, asthma, pulmonary fibrosis, and lung cancer.
Figure 5: The improved percent protection of lungs cells (A549) in terms of increased SOD activity under the stimulation of tert-butyl hydroperoxide (t-BHP). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item.
Estimation ofserotoninlevel in human neuroblastoma (SH-SY5Y) cells
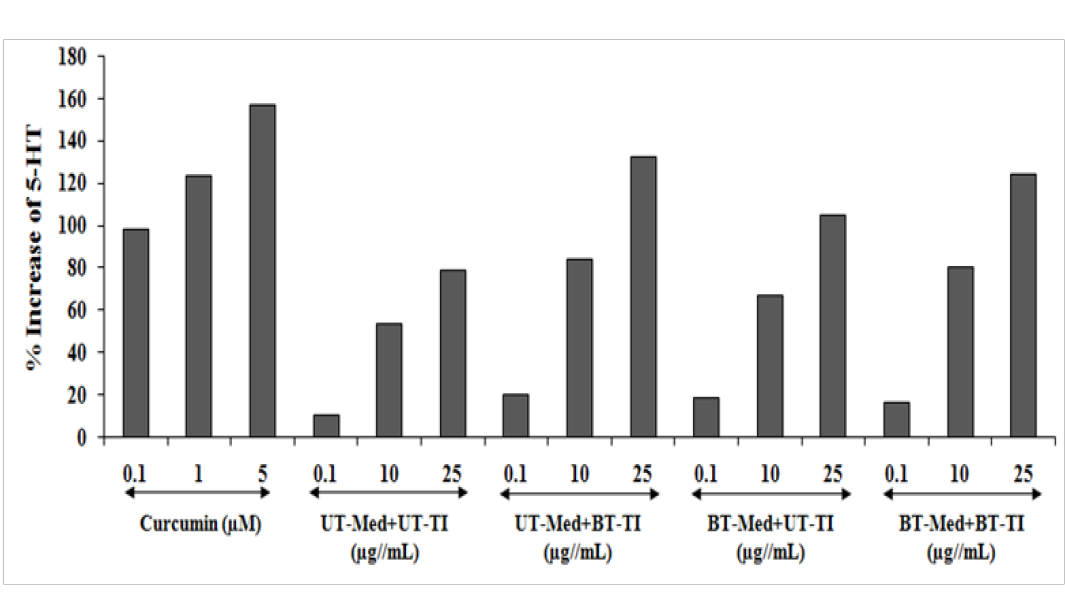
Serotonin assay was performed using SH-SY5Y cells and the effect of test formulation was assessed after 24 hours of treatment using ELISA assay. Serotonin activity was tested and the data is presented in the Figure 6. Curcumin was used a positive control, showed 98.2%, 123.5%, and 156.8% increased the level of serotonin at 0.1, 1, and 5 µg/mL respectively, compared to the vehicle control (VC) group. The data showed significant increased serotonin level by 56.4% and 68% at 10 and 25 µg/mL respectively, in the UT-Med + BT-TI, while significant increased serotonin by 75.7%, 23.8%, and 33.4% at 0.1, 10, and 25 µg/mL respectively, in the BT-Med + UT-TI, and 53.6%, 49.6%, and 57.2% improved serotonin level at 0.1, 10, and 25 µg/mL respectively, in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group (Figure 6). Thus, serotonin level was significantly improved in the entire tested group. Serotonin is supposed to be responsible for many neuropsychiatric disorders (viz. Alzheimer’s disease, cognitive health, loss of ability of thinking, depression, memory loss, etc.) along with various neuronal disorders like sleep, feeding, pain, sexual behavior, cardiac regulation, and cognition[56]. Our research study showed significant improved level of serotonin after treatment with the Biofield Energy Healing Treated test formulation that would be highly useful against various neurodegenerative diseases and improved brain functioning.
Figure 6: The effect of the test formulation on percent increase in 5-hydroxy tryptamine (5-HT) or serotonin in human neuroblastoma cells (SH-SY5Y). UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item
Effect of test formulation on vitamin D receptors (VDRs)
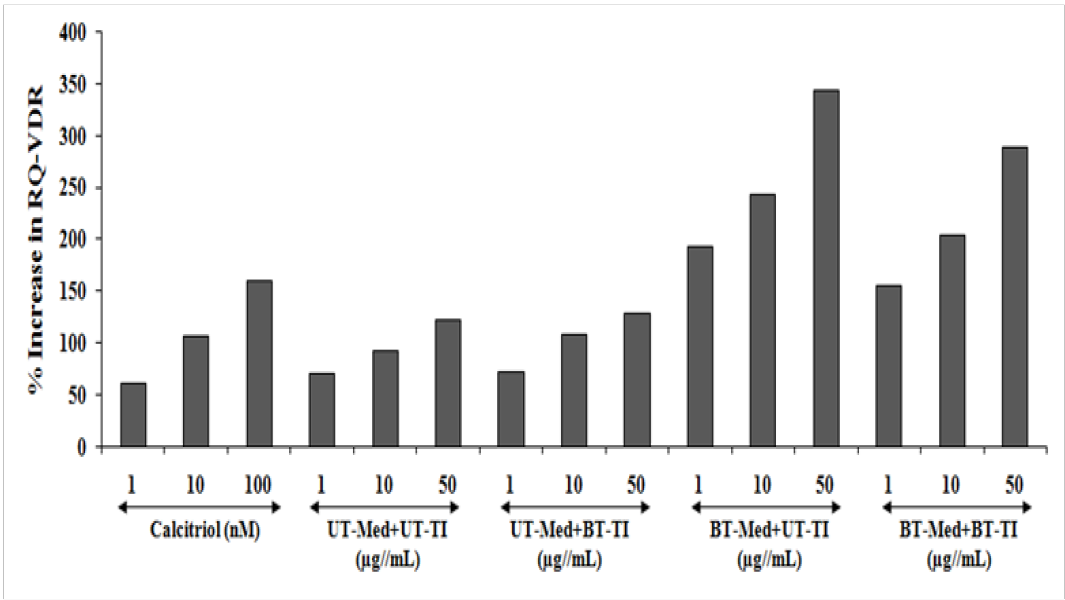
Human bone osteosarcoma cells (MG-63) was used for the estimation of VDR activity. The expression of VDRs was studies using the phenomenon of ligand binding through vitamin D active molecule, which was estimated using quantitative-polymerase chain reaction (qPCR) amplification. Using real time PCR, different VDR-relative threshold cycle (VDR-CT) values were obtained after complete amplification cycles using specific primer probes. Relative quantification (RQ) was calculated from the VDR-CT and house-keeping (HK)-CT values in MG-63 cells. The values after treated with the Biofield Energy Treated and untreated test formulation and positive control are represented in the Figure 7. Calcitriol was used as a positive control and the RQ of VDR was found to be increased in concentration-dependent manner by 61.3%, 107.1%, and 160.3% at 1, 10, and 100 nM, respectively. The experimental test groups showed increased RQ of VDR expression by 3.4%, 16.4%, and 5.1% in the UT-Med + BT-TI group at 1, 10, and 50 µg/mL respectively, while 173.4%, 161.2%, and 182% increased RQ of VDR at 1, 10, and 50 µg/mL respectively, in the BT-Med + UT-TI group, and increased RQ of VDR by 119.6%, 118%, and 137.1% at 1, 10 and 50 µg/mL respectively, in the BT-Med + BT-TI group as compared to the UT-Med + UT-TI group. In conclusion, VDR expression was significantly improved in MG-63 after treatment with the test formulation. Calcitriol was reported to bind with the VDRs and extensively regulates the calcium homeostasis, immunity, overall cellular growth, and differentiation[57]. Calcitriol controls various calcium metabolisms and play a vital role in improving quality of life and overall bone cell growth and development[58,59]. The Trivedi Effect® would be the best alternative treatment approach for bone related disorders.
Figure 7: Relative quantification (RQ) of vitamin D receptors (VDRs) gene in human bone osteosarcoma cells (MG-63).UT: Untreated; Med: Medium; BT: Biofield Treated; TI: Test item
Conclusion
Multiple organ health was analyzed using standard cell line assays. The safe concentrations of the test formulation was first analyzed using MTT assay, which showed that the test formulation was found safe and non-toxic against all the tested cell lines. Cytoprotective activity against t-BHP induced cell damage was tested using human cardiac fibroblasts cells (HCF), which showed restoration of cell viability by 133.4% (at 1 µg/mL), 65.7% (at 10 µg/mL), and 86.8% (at 10 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, BT-Med + BT-TI groups respectively, as compared to the untreated test group, while in HepG2 cells the maximum restoration of cell viability was 97.4%, 73%, and 39.4% at 1, 10, and 25 µg/mL respectively, in the UT-Med + BT-TI group, 43.4%, 85.7%, and 49.9% improved cellular restoration at 1, 10 and 25 µg/mL respectively, in the BT-Med + UT-TI, and 81.9% and 28.6% improved cellular restoration at 10 and 25 µg/mL respectively, in the BT-Med + BT-TI group as compared to the untreated test group. In A549 cells, cellular restoration was improved by 174.2%, 472.6%, 118%, and 279.2% at 0.1, 1, 10, and 25.5 µg/mL respectively, in the BT-Med + UT-TI group, while 1514%, 1654.8%, 449.8%, and 540.4% improved cellular restoration was reported at 0.1, 1, 10, and 25.5 µg/mL respectively, at BT-Med + BT-TI groups as compared to the untreated test group. Similarly, ALP activity in Ishikawa cells showed significantly increased ALP activity by 10.3% (10 µg/mL), 107.4% (50 µg/mL), and 60.9% (50 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI group, and BT-Med + BT-TI groups respectively, as compared to the UT-Med + UT-TI group. Similarly, ALP activity in MG-63 cells with maximum cellular protection was reported at 93.4% (50 µg/mL), 77.9% (1 µg/mL), 93.2% (50 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI group test groups, respectively, as compared with the untreated test group. LDH activity was significantly decreased and the data was presented in increased percentage cellular protection data, which showed maximum cellular protection by 35.9% (at 10 µg/mL), 69.3% (at 1 µg/mL), and 119.1% (at 1 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI group, and BT-Med + BT-TI groups respectively, as compared to the untreated test group.
ALT activity showed maximum improved cellular protection of HepG2 cells (decreased of ALT activity) by 23.8% (at 10 µg/mL), 98.1% (at 25.5 µg/mL), and 97.6% (at 25 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI group, and BT-Med + BT-TI groups respectively, as compared with the untreated test group. SOD activity was significantly increased by 73.5%, 109.8%, and 97.7% in the UT-Med + BT-TI, BT-Med + UT-TI group, and BT-Med + BT-TI groups respectively, at 0.1 µg/mL as compared with the untreated test group. Serotonin level was significantly increased in SH-SY5Y cells by 68% (at 25 µg/mL), 75.7% (at 0.1 µg/mL), and 57.2% (at 25 µg/mL) in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups respectively, as compared with the untreated test group. However, VDR expression was tested in MG-63 cells, which showed increased RQ of VDR by 16.4%, 182%, and 137.1% in the UT-Med + BT-TI, BT-Med + UT-TI, and BT-Med + BT-TI groups respectively, at 50 µg/mL as compared to the untreated test control group. Thus, this study concluded that the Biofield Energy based test formulation can improve the overall functioning of heart, liver, bones, neuronal, and lungs parameters against any oxidative stress or damage induced by free radicals. Biofield Energy Treatment (The Trivedi Effect®) can be used for the prevention of various types of cardiac disorders such as stroke, thromboembolic disease, congestive heart failure, congenital heart disease, peripheral artery disease, rheumatic heart disease, valvular heart disease, and venous thrombosis, etc. Besides, it would also protect against many hepatic disorders (cirrhosis, liver cancer, hemochromatosis, and Wilson disease), lungs disorders (asthma, chronic bronchitis, emphysema, cystic fibrosis, and pneumonia), and many immune disorders. In addition, this novel test formulation can also be utilized for organ transplants (i.e., kidney, liver, and heart transplants), hormonal imbalance, aging, and various inflammatory and immune-related disease conditions like Asthma, Aplastic Anemia, Graves’ Disease, Dermatitis, Diabetes, Parkinson’s Disease, Myasthenia Gravis, Ulcerative Colitis (UC), Atherosclerosis, etc. to improve overall health and Quality of Life.
Acknowledgements: Authors gratefully acknowledged to Trivedi Global, Inc., Trivedi Science, and Trivedi Master Wellness for their support. In addition, authors are thankful for the support of Dabur Research Foundation for conducting this study.
References
- 1. Langie, S.A., Lara, J., Mathers, J.C. Early determinants of the ageing trajectory. (2012) Best Pract Res Clin Endocrinol Metab 26(5): 613-626.
- 2. Johnson, T.E. Recent results: Biomarkers of aging. (2006) Exp Gerontol 41(12): 1243-1246.
- 3. Ryan-Harshman, M., Aldoori, W. Health benefits of selected minerals. (2005) Can Fam Physician 51(5): 673–675.
- 4. Rayman, M.P. The importance of selenium to human health. (2000) Lancet 356(9225): 233–241.
- 5. Beard, J.L., Connor, J.R. Iron status and neural functioning. (2003) Ann Rev Nutr 23: 41–58.
- 6. Coleman, C.I., Hebert, J.H., Reddy, P. The effects of Panax ginseng on quality of life. (2003) J Clin Pharm Ther 28(1): 5-15.
- 7. Das, L., Bhaumik, E., Raychaudhuri, U., et al. Role of nutraceuticals in human health. (2011) J Food Sci Technol 49(2): 173-183.
- 8. Czekanska, E.M., Stoddart, M.J., Richards, R.G., et al. In search of an osteoblast cell model for in vitro research. (2012) Europ Cells Mat 24: 1-17.
- 9. Schaefer, W.R., Fischer, L., Deppert, W.R., et al. In vitro-Ishikawa cell test for assessing tissue-specific chemical effects on human endometrium. (2010) Reprod Toxicol 30(1): 89-93.
- 10. Valavanidis, A., Vlachogianni, T., Fiotakis, K., et al. Pulmonary Oxidative Stress, Inflammation and Cancer: Respirable Particulate Matter, Fibrous Dusts and Ozone as Major Causes of Lung Carcinogenesis through Reactive Oxygen Species Mechanisms. (2013) Int J Environ Res Public Health 10(9): 3886-3907.
- 11. Bouma, M.E., Rogier, E., Verthier, N., et al. Further cellular investigation of the human hepatoblastoma-derived cell line HepG2: Morphology and immunocytochemical studies of hepatic-secreted proteins. (1989) In Vitro Cell Dev Biol 25(3): 267-275.
- 12. Bak, M.J., Jun, M., Jeong, W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. (2012) Int J Mol Sci 13(2): 2314-2330.
- 13. Molavi, B., Mehta, J.L. Oxidative stress in cardiovascular disease: molecular basis of its deleterious effects, its detection, and therapeutic considerations. (2004) Curr Opin Cardiol 19(5): 488-493.
- 14. Meneses, A., Liy-Salmeron, G. Serotonin and emotion, learning and memory. (2012) Rev Neurosci 23(5-6): 543-553.
- 15. Rohm, B., Holik, A.K., Somoza, M.M., et al. Nonivamide, a capsaicin analog, increases dopamine and serotonin release in SH-SY5Y cells via a TRPV1-independent pathway. (2013) Mol Nutr Food Res 57(11): 2008-2018.
- 16. Haussler, M.R., Whitfield, G.K., Haussler, C.A., et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. (1998) J Bone Miner Res 13(3): 325-349.
- 17. Dusso, A.S., Brown, A.J., Slatopolsky, E. Vitamin D. (2005) Am J Physiol Renal Physiol 289(1):F8-28.
- 18. Movaffaghi, Z., Farsi, M. Biofield therapies: Biophysical basis and biological regulations. (2009) Complement Ther Clin Pract 15(1): 35-37.
- 19. Barnes, P.M., Powell-Griner, E., McFann, K., et al. Complementary and alternative medicine use among adults: United States, 2002. (2004) Adv Data 343: 1-19.
Pubmed | Crossref | Others
- 20. Barnes, P.M., Bloom, B., Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. (2008) Natl Health Stat Report 12: 1-23.
- 21. Yang, P., Jiang, Y., Rhea, P.R., et al. Human Biofield Therapy and the Growth of Mouse Lung Carcinoma. (2019) Integr Cancer Ther.
- 22. Ross, C.L. Energy Medicine: Current Status and Future Perspectives. (2019) Glob Adv Health Med.
- 23. Fan, K.W. National Center for Complementary and Alternative Medicine Website. (2005) J Med Libr Assoc 93(3): 410-412.
Pubmed | Crossref | Others
- 24. Wisneski, L., Anderson, L. The Scientific Basis of Integrative Medicine. Boca Raton, FL: CRC Press; 2009: 205.
Pubmed | Crossref | Others
- 25. Trivedi, M.K., Tallapragada, R.M. A transcendental to changing metal powder characteristics. (2008) Met Powder Rep 63: 22-28, 31.
- 26. Trivedi, M.K., Nayak, G., Patil, S., et al. Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. (2015) Ind Eng Manage 4: 161.
Pubmed | Crossref | Others
- 27. Trivedi, M.K., Branton, A., Trivedi, D., et al. Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica L.). (2015) J Food Nutrition Sciences 3(6): 245-250.
Pubmed | Crossref | Others
- 28. Trivedi, M.K., Branton, A., Trivedi, D., et al. Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. (2015) J Clin Med Genom 3: 129.
Pubmed | Crossref | Others
- 29. Trivedi, M.K., Patil, S., Shettigar, H., et al. Evaluation of biofield modality on viral load of Hepatitis B and C viruses. (2015) J Antivir Antiretrovir 7: 083-088.
Pubmed | Crossref | Others
- 30. Trivedi, M.K., Patil, S., Shettigar, H., et al. Phenotypic and biotypic characterization of Klebsiella oxytoca: An impact of biofield treatment. (2015) J Microb Biochem Technol 7: 203-206.
Pubmed | Crossref | Others
- 31. Nayak, G., Altekar, N. Effect of biofield treatment on plant growth and adaptation. (2015) J Environ Health Sci 1: 1-9.
- 32. Branton, A., Jana, S. The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. (2017) Inter J Clinical and Developmental Anatomy 3: 9-15.
Pubmed | Crossref | Others
- 33. Branton, A., Jana, S. The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. (2017) American Journal of Clinical and Experimental Medicine 5: 138-144.
Pubmed | Crossref | Others
- 34. Kinney, J.P., Trivedi, M.K., Branton, A., et al. Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. (2017) American Journal of Life Sciences 5(2): 65-74.
Pubmed | Crossref | Others
- 35. Singh, J., Trivedi, M.K., Branton, A., et al. Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. (2017) American Journal of Pharmacology and Phytotherapy 2(1): 1-10.
Pubmed | Crossref | Others
- 36. Trivedi, M.K., Branton, A., Trivedi, D., et al. A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. (2017) Inter J Bioorganic Chemistry 2(3): 135-145.
Pubmed | Crossref | Others
- 37. Trivedi, M.K., Patil, S., Shettigar, H., et al. The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. (2015) J Integr Oncol 4(3): 141.
Pubmed | Crossref | Others
- 38. Anagnos, D., Trivedi, K., Branton, A., et al. Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. (2018) Inter J Biomedical Engineering and Clinical Science 4: 6-14.
Pubmed | Crossref | Others
- 39. Lee, A.C., Trivedi, K., Branton, A., et al. The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). (2018) Inter J Nutrition Food Sciences 7(1): 30-38.
Pubmed | Crossref | Others
- 40. Stutheit, M.E., Trivedi, K., Branton, A., et al. Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. (2018) American Journal of Life Sciences 6: 13-21.
Pubmed | Crossref | Others
- 41. Alía, M., Ramos, S., Mateos, R., et al. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). (2005) J Biochem Mol Toxicol 19(2): 119-128.
- 42. Vargas-Mendoza, N., Madrigal-Santillán, E., Morales-González, A., et al. (2014) Hepatoprotective effect of silymarin. World J Hepatol 6(3): 144-149.
- 43. Alía M, Ramos S, Mateos R, Bravo L, Goya L (2005) Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2). J Biochem Mol Toxicol 19(2): 119-28.
- 44. Videla, L.A. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. (2009) World J Hepatol 1(1): 72-78.
Pubmed | Crossref | Others
- 45. Li, S., Tan, H.Y., Wang, N., et al. The role of oxidative stress and antioxidants in liver diseases. (2015) Int J Mol Sci 16(11): 26087-26124.
- 46. Cheresh, P., Kim, S.J., Tulasiram, S., et al. Oxidative stress and pulmonary fibrosis. (2013) Biochim Biophys Acta 1832(7): 1028-1040.
- 47. Lu, L.Y., Ou, N., Lu, Q.B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. (2013) Sci Rep 3: 3169.
- 48. Atkins, G.J., Findlay, D.M., Anderson, P.H., Morris, H.A. Vitamin D (Third Edition) (2011) Target Genes: Bone Proteins 1: 411-424.
Pubmed | Crossref | Others
- 49. Emami, A., Larsson, A., Petrén-Mallmin, M., et al. Serum bone markers after intramedullary fixed tibial fractures. (1999) Clin Orthop Relat Res 368: 220-229.
- 50. Burgner, J.W., Ray, W.J. On the origin of the lactate dehydrogenase induced rate effect. (1984) Biochemistry 23(16): 3636-3648.
- 51. Valvona, C.J., Fillmore, H.L., Nunn, P.B., et al. The regulation and function of lactate dehydrogenase A: Therapeutic potential in brain tumor. (2015) Brain Pathol 26(1): 3-17.
- 52. Kopperschlager, G., Kirchberger, J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. (1996) J Chromatogr B Biomed Appl 684(1-2): 25-49.
- 53. Pratt, D.S., Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. (2000) N Engl J Med 342(17): 1266-1271.
- 54. Mathiesen, U., Franzen, L., Fryden, A., et al. The clinical significance of slightly to moderately increased liver transaminase values in asymptomatic patients. (1999) Scand J Gastroenterol 34(1): 85-91.
- 55. Birben, E., Sahiner, U.M., Sackesen, C., et al. Oxidative stress and antioxidant defense. (2012) World Allergy Organ J 5(1): 9-19.
- 56. Meltzer, C.C., Smith, G., DeKosky, S.T., et al. Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. (1998) Neuropsychopharmacology 18: 407-430.
- 57. Haussler, M.R., Whitfield, G.K., Haussler, C.A., et al. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. (1998) J Bone Miner Res 13(3): 325-349.
- 58. Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. (2005) J Nutr 135(2): 317-322.
- 59. Carlberg, C., Molnar, F. Current status of vitamin D signaling and its therapeutic applications. (2012) Curr Top Med Chem 12(6): 528-547.