Role of vitamin D receptor (VDR) genetic polymorphism in onset of type 2 diabetes mellitus
Fatma Hussain2, Sumera Shaheen1, Sobia Aleem1, Sadia Aslam1
Affiliation
1Department of Biochemistry, Government College Women University, Faisalabad
2Department of Biochemistry, University of Agriculture, Faisalabad
Corresponding Author
Naila Abdul Sattar, Department of Biochemistry, Government College Women University, Faisalabad, E-mail: uaf_naila_sattar@yahoo.com, dr.naila.sattar@gcwuf.edu.pk
Citation
Naila, A. S., et al. Role of Vitamin Gene Receptor Polymorphism in Onset of Type 2 Diabetes Mellitus (2019) Lett Health Biol Sci 4(1): 7-15.
Copy rights
© 2019 Naila, A. S. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
T2DM; VDR polmorphism; Pathogenesis
Abstract
Numerous studies disclosed the independent role of VDR genetic polymorphisms involved in pathogenesies of various metabolic disorders like type 2 diabetes mellitus in different populations, however no any conclusive or even key study conducted on South Asian population especially Pakistani population except[1] on Indian population. Worldwidley, vitamin D defeciency and type 2 diabetes mellitus (T2DM) are two interlated and most common health problems. Such interlationshipis involvedcomplex inheritance pattern.The polymorphisms of various genes including vitamin D receptor (VDR) might affect genetic susceptibility of T2DM by developing malfunctioning of beta pancreatic cells or insulin resistance. Geneticarchitecture of T2DM is different among various ethnic populations. The present review will focus on concept that polymorphism of VDR gene may has role in susceptibilty of onset of T2DM and its pathogenesises.
Introduction
Diabetes mellitus is endocrinological health issue which is reaching epidemic extents globally. Such increase in T2DM prevalence is endorsed by various environmental and genetic factors. Financial impact of T2DM is incalculably arduous as approximate anticipated cost of Pakistan’s health centers willbe up to $490 billion in coming ten years[2]. Common pathphysiological complications of T2DM are micro and macro vascular diseases, nephropathy and neuropathy. Such complications may develop due to sustained hyperglycemia, insulin resistance and dysfunction of beta cells[3-5] Insulin resistance established in early development to type 2 diabetes mellitus. With passage of time the compensatory reaction of pancreatic beta cells become weakened and leads to sustained hyperglycemia[6-8].
Risk Factors: Both acquired and genetic factors are measured to have imperative roles in onset T2DM. Firstdegree relatives and monozygotic twins affected 50 % to T2DM due to heritability[9,10]. More than 50 various genes are related to T2DM[11,12]. Obesity and low socioeconomic are considered major risk factor for T2DM after genetic menaces[13,14].
Discussion
Beta cell dysfunction and Insulin sensitivity: Both dysfunction of pancreatic beta cells and lack of insulin sensitivity take part in the development of T2DM, it is certainly dysfunction of beta cells that is serious to the progression of the diseases diabetes mellitus cannot arise deprived of impairment of the insulin production[15-23].

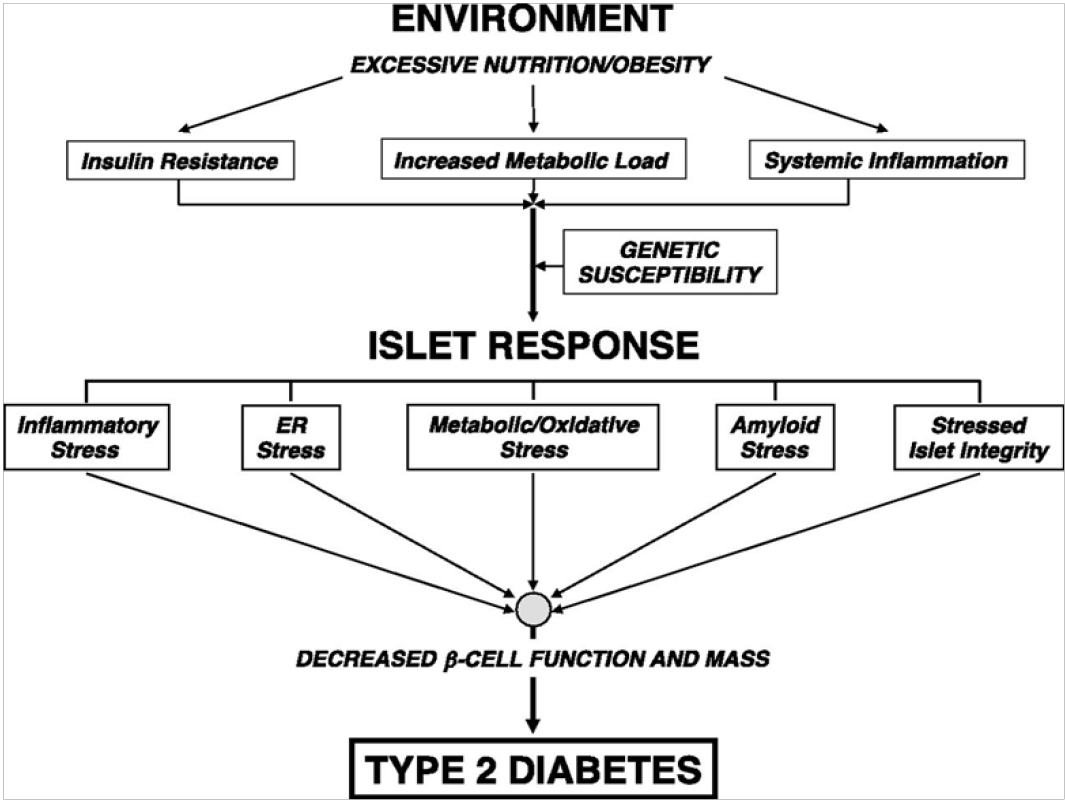
Few plausible menaces which have currently been recognized involve glucotoxicity to the beta cell which would be the outcome of prolonged enduring hyperglycemia, lipotoxicity caused by elevated levels of free fatty acid that often coexist in people with higher adiposity and lack of insulin[24], oxidative stress and long-lasting subclinical inflammation[25], additional visceral adipose tissue[26,27], lack of insulin sensitivity of pancreatic beta cells[25] and low adiponectin[28,29]. Family histories as well as genetics arealso considered to take part in defining risk of dysfunction of pancreatic beta cells[30,31].
Vitamin D: Vitamin D is necessary for the homeostasis of calcium to prevent rickets and osteomalacia[32]. In teenagers, hypovitaminosis D predisposes to rickets, a disorder of bones characterized by poor mineralization of skeletal tissues causing retardation of growth and deformities of skeletal comprising bony projections with rib cage and deformed legs or collided knees. In old age people, hypovitaminosis D develops osteomalacia, a defect in mineralization producing tender bone pain and weakness of muscles[34,35].

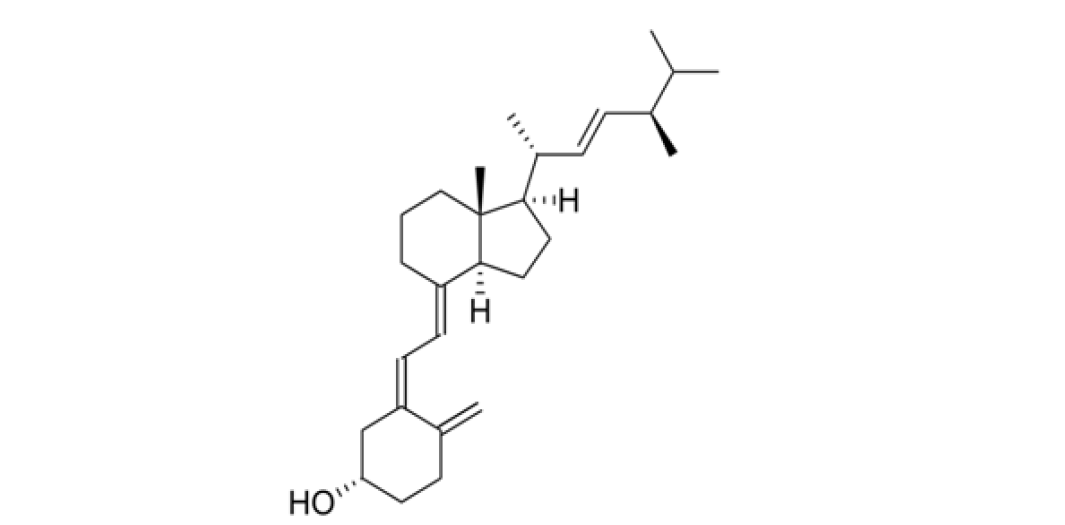
Figure 1: Chemical structure of Vitamin D
Sources of Vitamin D: Mostly the cutaneous production of vitamin D after the exposure to sunlight is considered to be main source in which mainly ultraviolet B (UVB) radiation of sunlight (290 to 315 nm) commence the photochemical reaction[36]. Furthermore other than this endogenous synthesis, humans can also get vitamin D through food supply. Almost all food sources are initiated from ultra violet radiation of plant ergosterol and sterol, present in plasma membranes of both fungus and yeast and synthesizing vitamin D2 or ergocalciferol and vitamin D3 or cholecalciferol by animal sources[37,38].
However vitamin D2 is less effective as compared to vitamin D3 in raising serum concentrations of vitamin D[39] and suggested that vitamin D3 can be employed for clinical as well as nutritional demands[40].
Metabolism of Vitamin D: Cholcalciferol enters blood stream through binding to protein known as vitamin D binding protein (DBP) and undergoes hydroxylation through cytochrome P450 enzyme hydroxylase (CYP2R1) to 25-hydroxyvitamin D also known as calcidiol in liver. Calicidiol is the chief circulating type of this vitamin in body[41,42,44]. Subsequently, 25-hydroxyvitamin D is then transported to kidneys where 1-alpha-hydroxylaseconverts 25-hydroxyvitamin D into active metabolite of vitamin D that is 1, 25-dihydroxyvitamin D or calcitriol[43,45-47].

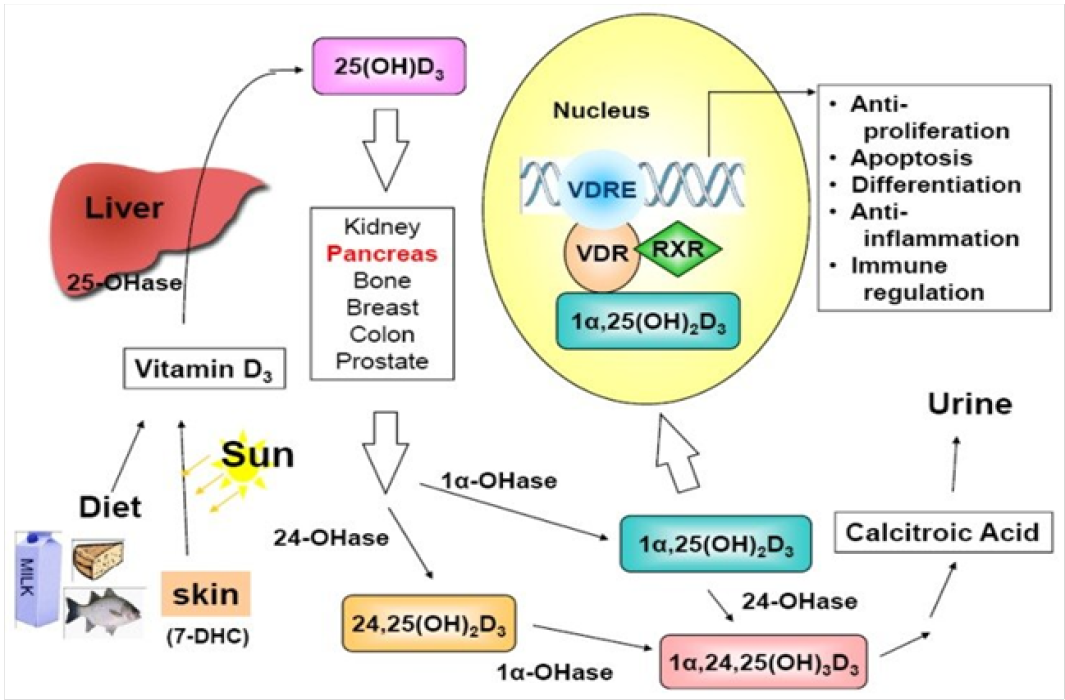
Figure 2: Metabolism of Vitamin D a schematic flow sheet
About 2-3% of human genome is indirectly or directly regulated through vitamin D coordination[48]. Furthermore, it has been established that locally produced dihydroxy vitamin D may control more than 2000 genes which take part in various processes comprising immunity, cell growth, inflammation and cell proliferation[49,50]. The genomic function of vitamin D requires the joining of calcitriol to strong affinity receptor, Vitamin D Receptor (VDR). It is a member of superfamily of the nuclear hormone receptors which acts as a ligand activated transcription factor[51]. However, the VDR can be present in organs involve in metabolism of calcium and homeostasis constituting the bone, intestine, parathyroid glands and kidney. VDRs have also been recognized in many other tissues; breast, heart, colon, pancreas and prostate[52,53].
Moreover, besides genomic function, vitamin D also facilitates a rapid non-genomic function that is found through the attachment of vitamin D to a cell membrane VDR. Such non-genomic functions of vitamin D are vital in nuclear transcription activity and membrane associated actions, such as elevating calcium uptake, secretion of calcium from its intracellular stores and excitement of protein kinase C action[50,54].
Factors affecting vitamin D levels
|
Factor |
Affecting Mechanism |
|
Solar zenith angle[55] |
Duration of day time and latitude |
|
Dark skin coloration[53] |
skin high melanin component contends along 7-dehydrocholesterol through absorbs UV photons |
|
Consumption of Sunscreen[36,54] |
decrease dermal synthesis of vitamin D |
|
High rate of Adiposity[57,58] |
Low vitamin D due to repossession of fat soluble vitamins in adipocytes, |
|
Fibrosis, cystic, celiac and Chron’s diseases[61-63] |
Reduced bioavailability of vitamin D due to diminished capability to absorb this vitamin |
|
Genetic influences[64,65] |
VDR gene and other genes polymorphisms |
Vitamin D and type 2 diabetes mellitus: The actions of vitamin D upon skeletal health is indicating its significant action in many other disorders and health conditions including; cardiovascular diseases, cancer, autoimmune disorders, and T2DM[55-66]. Deficiency of vitamin D is related with reduced insulin secretion and supplementation of vitamin D reestablished normal insulin secretion[67,68]. Moreover, seasonal changes in insulin and glucose concentrations[69,70], as well as seasonal changes in diagnosis and management of T2DM have been noted. There is more diagnosis and lesser glycemic control during winter as compared to summer[71]. Furthermore, most case control research outcomes have also documented that T2DM patients or those with impaired tolerance of glucose are expected to have a poor concentrations of vitamin D than to those without T2DM[72,73].
Only two randomized control trials suggesting the effects of this vitamin supplementation on incidence of T2DM are available in literature[74,75], as most of these trials stated the effects of vitamin D on insulin resistance, glycemic control and insulin secretion in preliminary inferences and determined no statistically significant action of vitamin D supplementation [400 IU / day[74]; 800 IU / day[75] on occurrence of diabetes mellitus after three to seven years of follow-up.
Association of vitamin D and insulin resistance: A number of researches have examined the function of vitamin D in initial pathophysiological conditions underlying T2DM, especially lack of insulin sensitivity and pancreatic beta cell dysfunction. Asignificant role of this vitamin with lack of insulin and pancreatic beta cell function has been derived. Uneven outcomes have also been stated in cross sectional analyses considering the relationship of vitamin D with function of beta cell, indicating a positive association[76-78] or no significant relationship[79-84].
Mechanism: Numerous prospective processes have been recommended to describe the relationship of vitamin D to T2DM and its associated manifestations. Vitamin D can directly increase action of insulin for the transportation of glucose via exciting the expression of insulin receptors[85], as (vitamin D response element) VDRE is located in promoter region of insulin receptor gene[86]. Vitamin D cannot directly affect lack of insulin sensitivity by maintaining intracellular processing of insulin mediated by the regulation of calcium pool[87,88]. Elevated intracellular calcium may stop insulin target cells to sense sharp intracellular fluctuations in calcium which are necessary for insulin action involving glucose transport[67,89]. This is also significant to note that initial determinants of peripheral sensitivity of insulin, skeletal muscle and adipocytes, express the VDR[50,90] and like sensitivity of insulin, the expression of VDR decline in skeletal muscle with age[91]. In addition, the expression of vitamin D α -hydroxylase observed in various tissues of wistar rats[92], initiating the local synthesis of vitamin D. With respect to pancreatic beta cell function, calcitriol can apply direct effects by binding of its active form in circulation to the beta cell VDR[93,94]. Instead, activation of this vitamin could happen within the pancreatic beta cell by vitamin D 1-α-hydroxylase that has been designated to express in pancreatic beta cells[95]. Furthermore, assuming the occurrence of VDRE in insulin gene promoter region, this may interpret the transcriptional activation of insulin gene through vitamin D[96]. Vitamin D can also employ indirect effect on beta cell function by maintaining extracellular calcium and its flux through the beta cell[97] as secretion of insulin is a calcium dependent phenomenon[35]. Based on the relationship between T2DM and systemic inflammation[98] vitamin D may also improve the sensitivity of insulin and promote the function of beta cell by regulating the generation and actions of cytokines[99]. However, limited data have described the association between vitamin D and T2DM[99,100].
Role of genetics: Genetic variations can explain discrepancies in the literature with respect to the relationship of vitamin D to T2DM. Much research has been focused on various genotypes associated to the VDR, vitamin D binding protein (DBP) and vitamin D-1-α-hydroxylase. Polymorphisms that have been recognized in VDR gene, specifically ApaI, TaqI, FokI and BsmI may be related with T2DM, lack of insulin sensitivity and dysfunction of pancreatic beta cell. However, recent evidences are limited and their outcomes have been inconsistent. Studies have found imperative relationships of specific VDR polymorphisms with higher lack of insulin sensitivity[101-105] and insulin secretion[106-108]. Though, most of these researches have focused on Caucasian populations and have employed surrogate measures of beta cells functions and lack of insulin based during fasting. With regard to T2DM specifically, Ortlepp et al [109]. Observed a greater prevalence of T2DM among those with a certain BsmI genotype for VDR gene as compared to those deprived of this genotype. Some case-control studies described no significant variations in frequencies of genotype for different VDR genes in T2DM versus controls[109-115]. Therefore, further investigation into association between VDR polymorphisms and risk of T2DM is warranted predominantly in various ethnic populations. Genetic polymorphisms of the vitamin binding protein have been recognized suggesting an association of these polymorphisms and enhanced risk of T2DM[116] and lack of insulin sensitivity as calculated through fasting glucose or levels of insulin[117,118]. However, another gene related to vitamin D studied for a possible association with T2DM is vitamin D-1-α-hydroxylase (CYP1alpha), it is accountable for the change of hydroxyvitamin D to dihydroxyvitamin D (calcitriol). So far, single study has been done to date[112], which suggested no significant polymorphism in CYP1alpha gene in T2DM patients versus controls in the Polish population. Hence, significant association of specific genotype of CYP1alpha gene with T2DM was observed in obese subgroup. However, precise mechanism of this finding was not clear. Earlier, Jorde et al[119]. suggested that no significant relationship of T2DM exist with many Single Nucleotide Polymorphisms (SNP) linked with serum vitamin D level.
Vitamin D receptor polymorphisms: Four allelic variants of vitamin D receptor gene have been recognized: ApaI, FokI, BsmI and TaqI[72]. The functions of these vitamin D receptor polymorphisms have been comprehensively studied in T2DM patients[51]. Polymorphism genotype ApaI of VDR gene showed relationship to the insulin secretion in Bangladeshi population, which are at high risk of T2DM with higher prevalence of hypovitaminosis D. A correlation of ApaI polymorphism with fasting blood glucoselevel and intolerance of glucose was evident among those people who had diabetes symptoms at pre-diagnosis stage. Ogunkolade et al[51]. Illustrated a positive relationship between the BsmI (genotype bb) and TaqI (genotype TT) polymorphisms with decreased insulin secretory potential. Speer et al[120]. Proposed that obese T2DM patients have greater levels of C- peptide and VDR polymorphism of BsmI allele (BB-genotype) indicative of their probable role in pathogenesis of T2DM. Insufficiency of vitamin D was measured in these subjects and polymorphism of TaqI was an element related to insulin secretion. Though, there is strong evidence of link between T2DM and VDR polymorphism; conflicting results among different populations are reported[112].
In T2DM, the vitamin D receptor gene polymorphism of allele ApaI (aa genotype) was related with impaired secretion of insulin in Caucasian population, thus this population hada higher risk of developing T2DM[102]. Contrary to that, VDR gene polymorphisms of alleles FokI, TaqI, ApaI and BsmI had no noteworthy association with T2DM in a case control research within Bangladeshi population by Islam et al[121]. Insulin sensitivity was significantly decreased in T2DM cases of Bangladeshi origin.
It was concluded by Sung et al[122]. Those distributions of VDR gene alleles of the four SNPs (BsmI, TaqI, Tru9I and ApaI) were same in T2DM patients and controls. These evidences supporting or opposing a relationship of vitamin D receptor genotypes with menace of T2DM are conflicting. Polymorphisms present in intron 8 (BsmI) and exon 9 (TaqI) of vitamin D receptor gene had substantial linkage with type 2 diabetes mellitus, while distribution as well as frequency of genotype FokI(exon 2) and ApaI(exon 8) of the VDR were significantly similar in T2DM patients and healthy people. These results confirmed the previous inferences that VDR gene genotypes BsmI as well as TaqI polymorphisms are related with onset of type 2 diabetes mellitus[120,123]. Furthermore, Al-Daghri et al[124]. Explained that BsmI and TaqI single nucleotide polymorphisms that are significantly more common in T2DM patients were allied with elevated levels of cholesterol and lower levels of High Density Lipoprotein (HDL) cholesterol. However such results are yet not unambiguous as other researchers failed to demonstrate analogous relationship between FokI, ApaI, BsmI and TaqI polymorphisms and onset of type 2 diabetes mellitus in Indians[103], Turkish[114], Polish[125] and American populations[126]. The genetic polymorphism of VDR appears to be a significantly important genetic component in the onset of T2DM. Taking this the predisposition risk factor, it is also noted that TaqI and BsmI were markedly associated with T2DM in northern Indians[127]. In another study conducted on local population found a significant relationship between the SNP rs7968585 of VDR and risk of T2DM with possible in mysocardial infraction with limited idea of association about interactions between above polymorphism and vitamin D serum levels[128]. Although no significant association found in genotype and allele frequencies among diabetic complications were having patients of post menopause and healthy subjects regarding polymorphism of VDR except FokI in coronary heart disease.
The reasons of these discrepancies might be elucidated by the differences in genetic background among ethnic groups. An overview of VDR gene of the significant allelic variations associated to T2DM. Although summary depicted below is scarce and not compatible, nonetheless it portrays a possible association between VDR gene, metabolism of vitamin D and T2DM etiology / traits.
In spite of that it is widely acknowledged that not only genetics with poor unhealthy lifestyle have an intense impact on the progression of T2DM, many advance studies adjoining epigenetics which exposure of the developing embryo to different intrauterine abnormal environmental situations; gestational diabetes with at high risk of developing T2DM. The methylation of CpG cells of pancreas may be inheritable, epigenetic activity taking place in the progressing fetal genetic makeup may effect in long-term effects on metabolic management. Therefore, the persuade that epigenetic processes exert may be massivelysignificant not only as a way by which ecological factors impact progress of T2DM but also in its function in establishing a risk profile for developing T2DM even before birth[126,127].
Conclusion
The prevalence of T2DM is intensely increasing, both in Pakistan and worldwide. Furthermore, assuming the lack of correct diagnosis of vitamin D deficiency and multi-system implicationsin most populations are at greater risk of having inadequate levels of serum vitamin D, with over 75% people having vitamin D deficiency while 18% reported insufficient vitamin D levels[125]. Evolving evidence proposes a prospective role for this vitamin in risk of T2DM as well as its underlying pathophysiological complications, specifically lack of insulin sensitivity and dysfunction of beta cell. However, many epidemiological, interventional and biological researches have suggested a probable relationship of vitamin D to lack of insulin sensitivity and function of beta cell, although these evidences have been inconsistent. In recent years, a number of polymorphisms, such as BsmI and FokI have been observed in the vitamin D receptor genes which are able to change the function of VDR protein, while other polymorphisms in VDR gene found through variation of alleles in sites of restriction enzyme are TaqI and ApaI. The genetic background of T2DM remains unclear. However, it is suggested that the vitamin D receptor gene is an innovative candidate gene responsible to the susceptibility to T2DM.
Acknowledgement
Government College Women University, Faisalabad, Pakistan, Higher Education Commission, Islamabad, Pakistan
Conflict of Interest
There is no conflict of Interest
References
- 1. Bid, H.K., Konwar, R., Aggarwal, C.G., et al. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. (2009) Indian J Med Sci 63(5): 187-194.
- 2. Hussain, M., Syed, B.S.N., Maqsood, A. K., et al. Direct cost of treatment of diabetes mellitus type 2 in Pakistan. (2014) Int J Pharm Sci 6(11): 261-264.
Pubmed| Crossref| Others
- 3. Ishaq, M., Ghulam, J.K., Sibgha, Z., et al. Prevalence of complications in type 2 diabetes mellitus patients. . (2013) Pak J Physiol 9: 2.
Pubmed| Crossref| Others
- 4. Sohial, M. Prevalence of diabetic retinopathy among type-2 diabetes patients in Pakistan - vision registry. (2014) Pak J Ophthalmol 30: 204-205.
Pubmed| Crossref| Others
- 5. Orozco, L. J., Buchleitner, A. M., Gimenez-Perez, G., et al. Exercise or exercise and diet for preventing type 2 diabetes mellitus. (2008) Cochrane Database Syst Rev 3: CD003054.
- 6. Van de, B. M., Morán, I., Ferrer, J., et al. Insights into β-Cell biology and type 2 diabetes pathogenesis from studies of the islet transcriptome. (2014) Genet Diabetes 23: 111-121.
- 7. Maedler, K. Beta cells in type 2 diabetes - a crucial contribution to pathogenesis. (2008) Diabetes Obes Metab 10(5): 408-420.
- 8. Zanuso, S., Jimenez, A., Pugliese, G., et al. Exercise for the management of type 2 diabetes: a review of the evidence. (2010) Acta Diabetol 47(1): 15-22.
- 9. Pierce, M., Keen, H., Bradely, C. Risk of diabetes in offspring of parents with non-insulin-dependent diabetes. (1995) Diabetes Med 12(1): 6-13.
- 10. Herder, C., Roden, M. Genetics of type 2 diabetes: pathophysiologic and clinical relevance. (2011) Eur J Clin Invest 41(6): 679-692.
- 11. Imamura, M., Maeda, S. Genetics of type 2 diabetes: the GWAS era and future perspectives. (2011) J Endocr 58: 723-739.
Pubmed| Crossref| Others
- 12. Wheeler, E., Barroso, I. Genome-wide association studies and type 2 diabetes. (2011) Brief Funct. Genomics 10(2): 52-60.
- 13. Fagard, R.H., Nilsson, P.M. Smoking and diabetes--the double health hazard. (2009) Prim Care Diabetes 3(4): 205-209.
- 14. Meigs, J.B Epidemiology of type 2 diabetes and cardiovascular disease: translation from population to prevention: the Kelly West award lecture 2009. (2010) Diabetes Care 33(8): 1865-1871.
- 15. Bloomgarden, Z.T. Insulin resistance: current concepts. (1998) Clin Ther 20(2): 216-231.
- 16. Kadowaki, T., Yamauchi, T., Kubota, N., et al. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. (2007) Novartis Found Symp 286: 164-176.
Pubmed| Crossref| Others
- 17. Shoelson, S.E., Donath, M. Y. Type 2 diabetes as an inflammatory disease. (2011) Nat Rev Immunol 11(2): 98-107.
- 18. Reaven, G., Tsao, P.S. Insulin resistance and compensatory hyperinsulinemia: the key player between cigarette smoking and cardiovascular disease? (2003) J Am Coll Cardiol 41(6): 1044-1047.
- 19. Lima, M. L., Cruz, T., Rodrigues, L.E., et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome-evidences for its relation to insulin resistance. (2009) Diabetes Res Clin Pract 83(2): 257-262.
- 20. Bethea, S.W., Nestler J. E. Comorbidities in polycystic ovary syndrome: their relationship to insulin resistance. (2008) Panminerva Med 50(4): 295-304.
Pubmed| Crossref| Others
- 21. Gronbaek, H., Thomsen, K.L., Rungby, J., et al. Role of nonalcoholic fatty liver disease in the development of insulin resistance and diabetes. (2008) Expert Rev Gastroenterol Hepatol 2(5): 705-711.
- 22. Gastaldelli, A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. (2011) Diabetes Res Clin Pract 1: 60-65.
- 23. Greenberg, A.S., McDaniel, M.L. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. (2002) Eur J Clin Invest 32: 24-34.
- 24. Bonora, E. Protection of pancreatic beta-cells: is it feasible? (2008) Nutr Metab Cardiovasc Dis 18(1): 74-83.
- 25. Hanley, A.J., Williams, K., Gonzalez, C., et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. (2003) Diabetes 52(2): 463-469.
- 26. Utzschneider, K.M., Carr, D.B., Hull, R.L., et al. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. (2004) Diabetes 53(11): 2867-2872.
- 27. Kharroubi, I., Rasschaert, J., Eizirik, D. L., et al. Expression of adiponectin receptors in pancreatic beta cells. (2003) Biochem Biophys Res Commun 312(4): 1118-1122.
- 28. Bacha, F., Saad, R., Gungor, N., et al. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. (2004) Diabetes Care 27(2): 547-552.
- 29. Marchetti, P., Lupi, R., Federici, M., et al. Insulin secretory function is impaired in isolated human islets carrying the Gly(972)-->Arg IRS-1 polymorphism. (2002) Diabetes 51(5): 1419-1424.
- 30. Marchetti, P., Del Prato, S., Lupi, R., et al. The pancreatic beta-cell in human Type 2 diabetes. (2006). Nutr Metab Cardiovasc Dis 1: 3-6.
Pubmed| Crossref| Others
- 31. Norman, A.W. Sunlight, season, skin pigmentation, vitamin D, and 25-hydroxyvitamin D: integral components of the vitamin D endocrine system. (1998) Am J Clin Nutr 67(6): 1108-1110.
- 32. Holick, M.F., Vitamin D: A millenium perspective. (2003) J Cell Biochem 88(2): 296-307.
- 33. Holick, M.F. Vitamin D: evolutionary, physiological and health perspectives. (2011) Curr Drug Targets 12(1): 4-18.
- 34. Holick, M.F., Chen, T.C., Lu, Z., et al. Vitamin D and skin physiology: a D-lightful story. (2007) J Bone Miner Res 2: 28-33.
- 35. Calvo, M.S., Whiting, S.J., Barton, C.N. Vitamin D fortification in the United States and Canada: current status and data needs. (2004) Am J Clin Nutr 80(6): 1710-1716.
- 36. Calvo, M.S., Whiting, S.J., Barton, C.N. Vitamin D intake: a global perspective of current status. (2005) J Nutr 135(2): 310-316.
- 37. Tripkovic, L., Lambert, H., Hart, K.., et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. (2012) Am J Clin Nutr 95(6): 1357-1364.
- 38. Vieth, R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. (2009) Anticancer Res 29(9): 3675-3684.
- 39. DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. (2004) Am J Clin Nutr 80(6): 1689-1696.
- 40. Strushkevich, N., Usanov, S.A., Plotnikov, A.N., et al. Structural analysis of CYP2R1 in complex with vitamin D3. (2008) J Mol Biol 380: 95-106.
- 41. Sakaki, T., Kagawa, N., Yamamoto, K.., et al. Metabolism of vitamin D3 by cytochromes P450. (2005) Front Biosci 10: 119-134.
- 42. Nykjaer, A., Dragun, D., Walther, D., An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. (1999) Cell 96(4): 507-515.
- 43. Breslau, N.A. Normal and abnormal regulation of 1, 25 (OH)2 D synthesis. (2008) Am J Med Sci 296(6): 417-425.
- 44. Segersten, U., Correa, P., Hewison, M., et al. 25-hydroxyvitamin D (3)-1alpha-hydroxylase expression in normal and pathological parathyroid glands. (2002) J Clin Endocrinol Metab 87(6): 2967-2972.
- 45. Omdahl, J.L., Morris, H.A., May, B..K. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. (2002) Ann Rev Nutr 22: 139-166.
- 46. Bouillon, R., Bischoff-Ferrari, H., Willett, W. Vitamin D and health: perspectives from mice and man. (2008) J Bone Miner Res 23(7): 974-979.
- 47. Nagpal, S., Na, S., Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. (2005) Endocr Rev 26(5): 662-687.
- 48. Norman, A. W. Vitamin D receptor: new assignments for an already busy receptor. (2006) Endocrinology 147(12): 5542-5548.
- 49. Ogunkolade, B.W., Boucher, B.J., Prahl, J.M., et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. (2002) Diabetes 51(7): 2294-2300.
- 50. Anderson, P.H., May B.K., Morris, H.A. Vitamin D metabolism: new concepts and clinical implications. (2003) Clin Biochem Rev 24(1): 13-26.
- 51. Clemens, T.L., Adams, J. S., Henderson, S..L., et al. Increased skin pigment reduces the capacity of skin to synthesis vitamin D3. (2009) Lancet 1(8263): 74-76.
- 52. Fleet, J.C. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin D: what are they and what do they mean? (2004) J Nutr 134(12): 3215-3218.
- 53. Kimlin, M.G. Geographic location and vitamin D synthesis. (2008) Mol Aspects Med 29(6): 453-461.
- 54. Matsuoka, L.Y., Ide, L., Wortsman, J., et al. Sunscreens suppress cutaneous vitamin D3 synthesis. (2000) J Clin Endocrinol Metab 64(6): 1165-1168.
- 55. Liel, Y., Ulmer, E., Shary, J., et al. Low circulating vitamin D in obesity. (1999) Calcif Tissue Int 43(4): 199-201.
- 56. Arunabh, S., Pollack, S., Yeh, S., et al. Body fat content and 25-hydroxyvitamin D levels in healthy women. (2003) J Clin Endocrinol Metab 88(1): 157-161.
- 57. Wortsman, J., Matsuoka, L.Y., Chen, T.C., et al. Decreased bioavailability of vitamin D in obesity. (2003) Am J Clin Nutr 72: 690-693.
- 58. Blum, M., Dolnikowski, G., Seyoum, E., et al. Vitamin D(3) in fat tissue. (2008) Endocrine 33(1): 90-94.
- 59. Lo, C.W., Paris, P.W., Clemens, T.L., et al. J. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. (2005) Am J Clin Nutr 42(4): 644-649.
- 60. Masuda, S., Okano, T., Osawa, K., et al. Concentrations of vitamin D-binding protein and vitamin D metabolites in plasma of patients with liver cirrhosis. (1999) J Nutr Sci Vitaminol (Tokyo) 35(4): 225-234.
- 61. Ishimura, E., Nishizawa, Y., Inaba, M., et al. Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D and 25- hydroxyvitamin D in non-dialyzed patients with chronic renal failure. (2000) Kidney Int 55(3): 1019-1027.
- 62. Arguelles, L.M., Langman, C.B., Ariza, A.J., et al. Heritability and environmental factors affecting vitamin D status in rural Chinese adolescent twins. (2009) J Clin Endocrinol Metab 94(9): 3273-3281.
- 63. Karohl, C., Su, S., Kumari, M., et al. Heritability and seasonal variability of vitamin D concentrations in male twins. (2010) Am J Clin Nutr 92(6): 1393-1398.
- 64. Boyd, A.E., Hill, R.S., Oberwetter, J. M., et al. Calcium dependency and free calcium concentrations during insulin secretion in a hamster beta cell line. (2006) J Clin Invest 77(3): 774-781.
- 65. Norman, A.W., Frankel, J.B., Heldt, A.M. et al. Vitamin D deficiency inhibits pancreatic secretion of insulin. (2004) Sci 209(4458): 823-825.
- 66. Clark, S.A., Stumpf W.E., Sar, M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. (20100029 Diabetes 30(5): 382-386.
- 67. Behall, K.M., Scholfield, D.J., Hallfrisch, J.G., et al. Seasonal variation in plasma glucose and hormone levels in adult men and women. (2004) Am J Clin Nutr 40(6): 1352-1356.
- 68. de Souza, C.J., Meier, A.H. Circadian and seasonal variations of plasma insulin and cortisol concentrations in the Syrian hamster, Mesocricetus auratus. (2007) Chronobiol Int 4(2): 141-151.
- 69. Doro, P., Benko, R., Matuz, M., et al. Seasonality in the incidence of type 2 diabetes: a population-based study. (2006) Diabetes Care 29(1): 173-173.
- 70. Pittas, A.G., Dawson-Hughes, B., Li, T., et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. (2006) Diabetes Care 29(3): 650-656.
- 71. Scragg, R., Sowers, M., Bell, C. serum 25-hydroxyvitamin D, diabetes and ethnicity in the third national health and nutrition examination survey. (2004) Diabetes Care 27: 2813-2818.
- 72. de Boer, I.H., Tinker, L.F., Connelly, S., et al. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. (2008) Diabetes Care 31(4): 701-707.
- 73. Avenell, A., Cook, J.A., MacLennan, G.S. and G. C., et al. Record trial group. Vitamin D supplementation and type 2 diabetes: a sub-study of a randomized placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438). (2009) Age Ageing 38(5): 606-609.
- 74. Boucher, B.J., Mannan, N., Noonan, K., et al. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. (1995) Diabetologia 38(10): 1239-1245.
- 75. Baynes, K.C., Boucher, B.J., Feskens, E.J., et al. Vitamin D, glucose tolerance and insulinaemia in elderly men. (1997) Diabetologia 40(3): 344-347.
- 76. Wu, T., Willett, W.C., Giovannucci, E. Plasma C-peptide is inversely associated with calcium intake in women and with plasma 25-hydroxy vitamin D in men. (2009) J Nutr 139(3): 547-554.
- 77. Orwoll, E., Riddle, M., Prince, M. Effects of vitamin D on insulin and glucagon secretion in non-insulin-dependent diabetes mellitus. (1994) Am J Clin Nutr 59(5): 1083-1087.
- 78. Chiu, K.C., Chu, A., Go, V.L., et al. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. (2004) Am J Clin Nutr 79(5): 820-825.
- 79. Gulseth, H.L., Gjelstad, I. M., Tierney, A.C., et al. Serum vitamin D concentration does not predict insulin ction or secretion in European subjects with the metabolic syndrome. (2010) Diabetes Care 33(4): 923-925.
- 80. Del Gobbo, L.C., Song, Y., Dannenbaum, D.A., et al. Serum 25-hydroxyvitamin D is not associated with insulin resistance or beta cell function in Canadian Cree. (2011) J Nutr 141(2): 290-295.
- 81. Rhee, S.Y., Hwang, Y.C., Chung, H.Y., et al. Vitamin D and diabetes in Koreans: analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2009. (2012) Diabet Med 29(8): 1003-1010.
- 82. Maestro, B., Campion, J., Davila, N., et al. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. (2000) J Endocr 47(4): 383-391.
Pubmed| Crossref| Others
- 83. Maestro, B., Davila, N., Carranza, M.C., et al. Identification of a Vitamin D response element in the human insulin receptor gene promoter. (2003) J Steroid Biochem Mol Biol 84(2-3): 223-230.
- 84. Draznin, B., Sussman, K., Kao, M., et al. The existence of an optimal range of cytosolic free calcium for insulin-stimulated glucose transport in rat adipocytes. (1987) J Biol Chem 262(30): 14385-14388.
- 85. Draznin, B. Intracellular calcium, insulin secretion and action. (1988) Am J Med 85(5A): 44-58.
- 86. Worrall, D.S., Olefsky, J.M. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. (2002) Mol. Endocrinol 16(2): 378-389.
- 87. Bischoff, H.A., Borchers, M., Gudat, F., et al. In situ detection of 1, 25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. (2001) Histochem J 33(1): 19-24.
- 88. Bischoff-Ferrari, H.A., Borchers, M., Gudat, F., et al. Vitamin D receptor expression in human muscle tissue decreases with age. (2004) J Bone Miner Res 19(2): 265-269.
- 89. Li, J., Byrne, M.E., Chang, E., et al. 1-alpha, 25-Dihydroxyvitamin D hydroxylase in adipocytes. (2008) J Steroid Biochem Mol Biol 112(1-3): 122-126.
- 90. Johnson, J.A., Grande, J.P., Roche, P.C., et al. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. (1994) Am J Physiol 267: 356-360.
- 91. Zeitz, U., Weber, K., Soegiarto, D.W., et al. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. (2003) J FASEB 17(3): 509-511.
- 92. Bland, R., Markovic, D., Hills, C.E., et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. (2006) J Steroid Biochem Mol Biol 89-90(1-5): 121-125.
- 93. Maestro, B., Davila, N., Carranza M.C., et al. Identification of a Vitamin D response element in the human insulin receptor gene promoter. (2003) J Steroid Biochem Mol Biol 84(2-3): 223-230.
- 94. Sergeev, I.N., Rhoten, W.B. 1, 25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. (1995) Endocrinol 136(7): 2852-2861.
- 95. Donath, M.Y., Shoelson, S.E. Type 2 diabetes as an inflammatory disease. (2011) Nat Rev Immunol 11(2): 98-107.
- 96. Pittas, A.G., Lau, J., Hu, F.B., et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. (2007) J Clin Endocrinol Metab 92(6): 2017-2029.
- 97. Cigolini, M., Iagulli, M.P., Miconi, V., et al. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. (2006) Diabetes Care 29(3): 722-724.
- 98. Chiu, K.C., Chuang, L.M., Yoon, C. The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. (2001) BMC Med Genet 2: 2.
- 99. Oh, J.Y., Barrett-Connor, E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo study. (2002) Metabolism 51(3): 356–359.
- 100. Ortlepp, J. R., Metrikat, J., Albrecht, M.., et al. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. (2003) Diabetes Med 20(6): 451-454.
- 101. Filus, A., Trzmiel, A., Kuliczkowska-Plaksej, J., et al. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. (2003) Aging Male 11(3): 134-139.
- 102. Tworowska-Bardzinska, U., Lwow, F., Kubicka, E., et al. The vitamin D receptor gene BsmI polymorphism is not associated with anthropometric and biochemical parameters describing metabolic syndrome in postmenopausal women. (2008) Gynecol Endocrinol 24(9): 514-518.
- 103. Hitman, G.A., Mannan, N., McDermott, M.F., et al. Vitamin D receptor gene polymorphisms influence insulin secretion in Bangladeshi Asians. (1998) Diabetes 47(4): 688-690.
- 104. Speer, G., Cseh, K.., Winkler, G., et al. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. (2001) Eur J Endocrinol 144(4): 385-389.
- 105. Ogunkolade, B.W., Boucher, B.J., Prahl, J.M., et al. Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. (2002) Diabetes 51(7): 2294-2300.
- 106. Ortlepp, J.R., Lauscher, J., Hoffmann, R., et al. The vitamin D receptor gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. (2001) Diabetes Med 18(10): 842-845.
Pubmed| Crossref| Others
- 107. Boullu-Sanchis, S., Lepretre, F., Hedelin, G., et al. Type 2 diabetes mellitus: association study of five candidate genes in an Indian population of Guadeloupe, genetic contribution of FABP2 polymorphism. (1999) Diabetes Metab 25(2): 150-156.
- 108. Ye, W, Z., Reis, A.F., Dubois-Laforgue, D., et al. VDR gene polymorphisms are associated with obesity in type 2 diabetic subjects with early age of onset. (2001) Eur J Endocrinol 145(2): 181-186.
Pubmed| Crossref| Others
- 109. Malecki, M.T., Frey, J., Moczulski, D., et al. VDR gene polymorphisms and association with type 2 diabetes mellitus in a polish population. (2003) Exp Clin Endocrinol Diabetes 111(8): 505-509.
- 110. Bid, H.K., Konwar, R., Aggarwal, C.G., et al. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. (2009) Indian J Med Sci 63(5): 187-194.
- 111. Dilmec, F., Uzer, E., Akkafa, F., et al. Detection of VDR gene ApaI and TaqI polymorphisms in patients with type 2 diabetes mellitus using PCR-RFLP method in a Turkish population. (2010) J Diabetes Complications 24(3): 186-191.
- 112. Vural, H.C., Maltas, E. RT-qPCR assay on the vitamin D receptor gene in type 2 diabetes and hypertension patients in Turkey. (2012) Genet Mol Res 11(1): 582-590.
- 113. Hirai, M., Suzuki, S., Hinokio, Y.., et al. Group specific component protein genotype is associated with NIDDM in Japan. (1998) Diabetologia 41(6): 742-743.
- 114. Baier, L. The Pima Diabetes Genes Group: Suggestive linkage of genetic markers on chromosome 4q12 to NIDDM and insulin action in Pima Indians: new evidence to extend associations in other populations. (1996) Diabetes 45(2): 30.
Pubmed| Crossref| Others
- 115. Szathmary, E.J. The effect of Gc genotype on fasting insulin level in Dogrib Indians. (2007) Hum Genet 75(4): 368-372.
- 116. Jorde, R., Schirmer, H., Wilsgaard, T., et al. Polymorphisms related to the serum 25-hydroxyvitamin D level and risk of myocardial infarction, diabetes, cancer and mortality. The Tromso Study. (2012) PLoS One 7(5): 37295.
- 117. Speer, G., Cseh, K., Winkler, G., et al. Vitamin D and estrogen receptor gene polymorphisms in type 2 diabetes mellitus and in android type obesity. (2001) Eur J Endocrinol 144(4): 385-389.
- 118. Islam, M.K., Siddhika, A., Manisha, D., et al. Association of vitamin D receptor gene Bsm1 (A>G) and Fok1 (C>T) polymorphism in the pathogenesis of impaired glucose tolerance in Bangladeshi subjects. (2014) J SMU Medical 1: 1-2.
Pubmed| Crossref| Others
- 119. Sung, C.C., Liao, M.T., Lu, K. C., et al. Role of vitamin D in insulin resistance. (2012) J Biomed Biotechnol: 634195.
- 120. Nosratabadi, R., Arababadi, M. K., V. A. Salehabad, A., et al. Polymorphisms within exon 9 but not intron 8 of the vitamin D receptor are associated with the nephropathic complication of type-2 diabetes. (2010) Int J Immunogenet 37(6): 493-497.
- 121. Al-Daghri, N.M., Al-Attas, O., Alokail, M.S., et al. Vitamin D receptor gene polymorphisms and HLA DRB1*04 cosegregation in Saudi type 2 diabetes patients. (2012) J Immunol 188(3): 1325-1332.
- 122. Masood, Z., Mahmood, Q., Khizar, T., et al. Vitamin D deficiency an emerging public health problem in Pakistan. (2010) JUMDC 1: 1-2.
Pubmed| Crossref| Others
- 123. Park J. H., Stoffers D. A., Nicholls R. D., et al. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. (2008) J Clin Invest 118(6): 2316–2324.
- 124. Hillier T. A., Pedula K. L., Schmidt M. M., et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. (2007) Diabetes Care 30(9): 2287–2292..
- 125. Ding G. L., Wang F. F., Shu J., et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. (2012) Diabetes 61(5): 1133–1142.
- 126. Malik R., Farooq, R., Mehta, P., et al. Association of Vitamin D Receptor Gene Polymorphism in Adults with Type 2 Diabetes in the Kashmir Valley. (2018) Can J Diabetes 42(3): 251-256.
- 127. Martinaityte, I., Jorde, R., Schirmer, H., et al. Correction: Genetic Variations in the Vitamin D Receptor Predict Type 2 Diabetes and Myocardial Infarction in a Community-Based Population: The Tromsø Study. (2016) PLOS ONE 11(9): e0163573.
- 128. Maia, J., da Silva, A.S., do Carmo, R.F., et al. The association between vitamin D receptor gene polymorphisms (TaqI and FokI), Type diabetes, and micro-/macrovascular complications in postmenopausal women. (2016) Appl Clin Genet 9: 131-136.












