Spindle Cell Lipoma: Rare Breast Mass Imaging Findings and-Differential Diagnosis
Quan Nguyen*, Nita Kommula, Safaraz Sadruddin, Angelica Robinson, Taylor Harmon, Barrett Riddle, Sara Ortiz, Suimin Qiu, David Pacheco, Usama Yassi, Sandra Hatch
Affiliation
Department of Radiology, UTMB, Texas, USA
Corresponding Author
Quan Nguyen, Department of Radiology, UTMB, Texas, USA; E-mail: qunguyen@utmb.edu
Citation
Nguyen, Q., et al. Spindle Cell Lipoma: Rare Breast Mass Imaging Findings and Differential Diagnosis. (2018) Int J Cancer Oncol 5(2): 60- 63
Copy rights
© 2018 Nguyen, Q. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
Spindle Cell Lipoma (SCL) of the breast is a rare variant of breast lipoma. It is most commonly observed in the subcutaneous soft tissues of the upper back and posterior neck in males. Our case details a 65-year-old female who presents for routine screening mammogram and was subsequently found to have an enlarging oval mass in the right breast. The patient was recalled for diagnostic mammogram including core biopsy which revealed mature adipose tissue with spindle cells and collagen. Although these lesions generally follow a benign course, the patient was advised to pursue a follow-up appointment with a breast surgeon as the lesion had doubled in size over the course of a few years.
Introduction
In December of 2017, a 65-year-old female presented to clinic for routine screening mammogram. Her a past medical history included coronary artery disease, type II diabetes, hypertension, hyperlipidemia, and uterine cancer. Screening mammogram revealed an oval mass in the upper-outer quadrant of the right breast. As such, the patient was assigned a BI-RADS category of 0 with a recommendation to compare to prior mammograms. Of note, the patient had previously undergone screening mammograms sporadically at an outside facility.
When comparison images were made available, it was noted that the oval mass in the upper-outer quadrant of the right breast had significantly increased in size from previous exams dating back to 2013. As such, the patient was recalled for a diagnostic workup and possible ultrasound guided biopsy.
Targeted ultrasound demonstrated a solid, echogenic mass at the site of the mammographic mass at 10 O’clock at a distance of 11 cm from the nipple measuring up to 3.7 cm in greatest dimension. No axillary lymphadenopathy was appreciated. These findings were assigned a BI-RADS category of 4B, moderate suspicion of malignancy, warranting a core biopsy. Histology revealed mature adipose tissue with spindle cells and collagen admixed with blood. The spindle cells were noted to be CD 34 positive, consistent with spindle cell lipoma.
Discussion
Spindle cell lipomas are rare, benign, slow growing tumors. Enzinger and Harvey first described spindle cell lipoma in 1975 as a benign lesion in which mature fat is replaced by collagen-forming spindle cells[1]. They most often occur in males between 40 – 70 years old in the subcutaneous tissue of the upper back, posterior neck, and shoulders[1-3]. Rarely it may occur at other sites, including the breast, oral cavity, scalp, extremities, and deep-seated muscles[4]. The overall incidence of spindle cell lipomas is 100 - fold greater in men than in women, but mammary spindle cell lipomas occur with equal frequency in males and females.
Review of the literature demonstrates a few case reports and one case series:
Case report of 28 year old woman (Pathology 1999;31:288)[5] .
Case report of 48 year old woman (Indian J Pathol Microbiol 2008;51:234)[6] .
Case report of breast lump in a 66-year-old female (Acta Cytol 2000;44:255)[7]
Case report of spindle cell lipoma of the breast found on mammography in a 53-year-old woman[8]
Case series of fine needle aspiration cytology of eight breast masses diagnosed cytologically as breast spindle cell tumors followed by wide excision biopsy[9].
The common clinical presentation for spindle cell lipomas is painless mobile palpable mass[10].
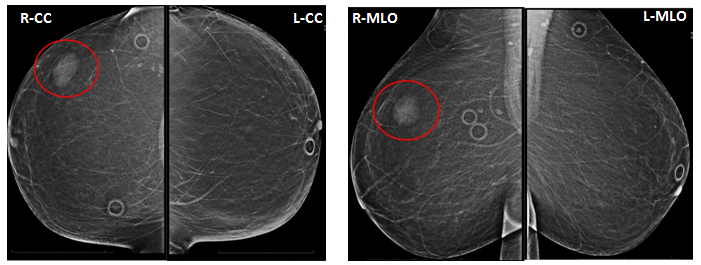
Mammography typically demonstrates a well-circumscribed mass without calcifications (Figure1). The differential for a well circumscribed mass on mammogram includes solid mass such as fibroadenoma, fatty mass such as lipoma, and cyst. The mammogram is not conclusive, and ultrasound is required to differentiate between mass versus cyst.
Figure 1: Screening Mammogram 12/2017
The patient presented for routine screening mammogram which revealed almost entirely fatty breasts. An oval density in the upper-outer quadrant of the right breast is seen on both CC and MLO views (red circle).
These findings were assigned a BI-RADS category of 0 with a request for comparison to prior films.
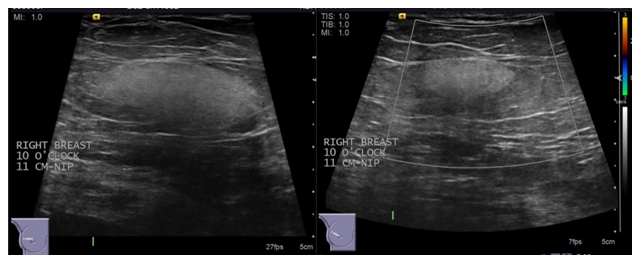
Ultrasound usually demonstrates a circumscribed hyperechoic mass with homogeneous echogenicity and minimal acoustic shadowing[9,11] (Figure 2). Hyperechoic lesions are not frequently seen on breast ultrasound, and when seen are almost always associated with benign pathologies that do not require further evaluation. However, malignancy such as invasive ductal carcinoma and metastasis may present with hyperechogenicity[12]. Hyperechoic breast lesions are not common[13], corresponding to 5.6% of ultrasound abnormalities, with high predictive value for benignity. Hyperechoic lesions only represent 0.6% of all biopsied lesions and only 0.4% of all malignant lesions[14]. Although hyperechoic breast masses are uncommon with high predictive value for benignity, evaluation should consider all the sonographic characteristics[15]. The most suspicious imaging findings such as non-circumscribed margins and posterior acoustic shadowing, along with mammographic and clinical correlation, should guide decision between conservative versus surgical management[16].
Figure 2: Comparison screening mammograms.
The patient had received prior mammograms at an outside facility which were retrieved for comparison. CC views from mammograms dated 2013, 2014, and the current mammogram dated 2017 are displayed side by side for comparison. The red arrow points to the progressively enlarging upper-outer quadrant oval mass in the right breast.
Given the progressive enlargement, diagnostic work up of the mass was recommended.
The MRI appearance of this lesion has yet to be described per review of current literature. The typical imaging for breast lipomas includes only mammogram and ultrasound as seen in this case. Given ultrasound guided biopsy provided pathology results for the patient in our case, no further imaging was indicated beyond ultrasound.
Figure 3: Ultrasound of the right breast.
Targeted ultrasound of the right breast reveals a solid mass at the site of the mammographic mass at 10 O’clock at a distance of 11 cm from the nipple that measures 37 x 34 x 16 mm. Characteristics of the mass include oval shape, circumscribed margins, echogenic echotexture, parallel orientation and no internal vascularity. No axillary lymphadenopathy was appreciated. Histology using core biopsy was recommended to further characterize the mass.
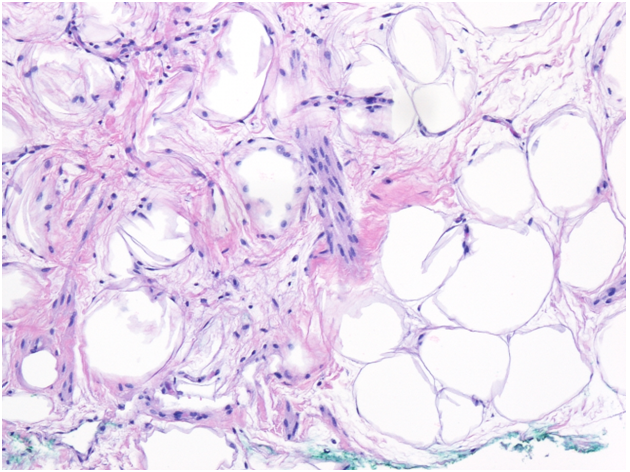
Pathology findings include uniform spindle cells, adipocytes, and collagen fibers[10]. It can be challenging to differentiate between benign and malignant lesions when they are encountered on core biopsy; therefore, surgical excision may be necessary[17].
Needle core biopsy of right breast (H&E 100x magnification). Sections show mature adipose tissue admixed with bland appearing spindle cells, blood vessels, and ropey collagen, consistent with spindle cell lipoma.
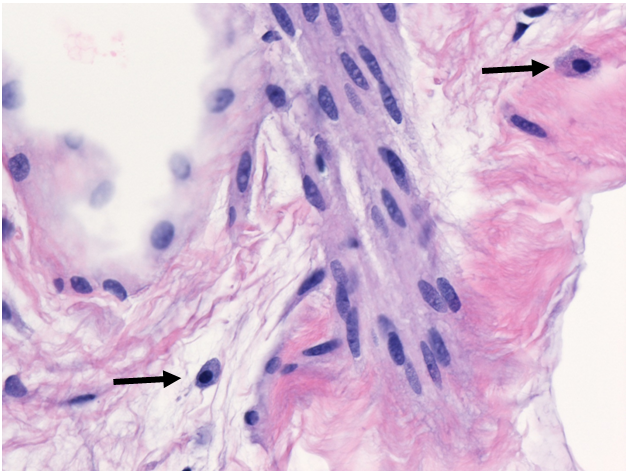
Needle core biopsy of right breast (H&E 400x magnification). Mast cells (arrows) are easily identified in spindle cell lipoma.
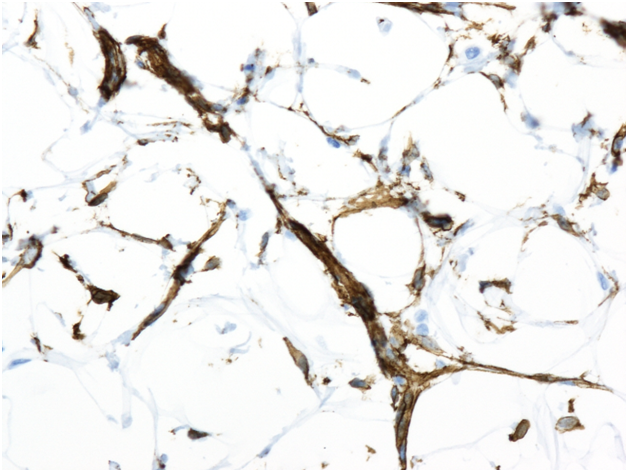
Needle core biopsy of right breast (Immunohistochemistry stain for CD34). Spindle cells stain strongly positive for CD34, helping differentiate it from similar entities such as angiolipoma, fibrolipoma sclerotic lipoma, and atypical spindle cell lipomatous tumor.
Soft-tissue tumors are first differentiated based on histologic morphology. Spindle cell lipoma (SCL) is a benign lipomatous tumor composed of mature adipocytes, bland fibroblast-like spindle cells, and thick “ropey” collagen. Prominent intermixed mast cells are also characteristic.
The bland fibroblast-like spindle cells are not a typical feature of the traditional lipoma and other benign lipoma variants. Inversely-spindle cell lipoma lacks the angiomatous component with fibrin thrombi of angiolipoma and the smooth muscle component of myolipoma. The spindle cells in SCL stain positive for CD34, like other benign lipomatous tumors but lack S100.
Other important entities in the differential include solitary fibrous tumor (SFT) and dermato fibrosarcoma prutuberans (DFSP) which are both spindle cell neoplasms that stain positive for CD34. SCL can be reliably differentiated from SFT and DFSP by the presence of mast cells, relatively lower cellularity, characteristic “ropey” collagen bundles, and absence of ectatic or hyalinized vessels[18].
This still leaves the possibility of an atypical lipomatous tumor (ALT). SCL is usually seen in the subcutaneous fat of posterior neck, upper back, and shoulder girdle. ALT has a predilection for deep tissue of the extremities (The term well-differentiated liposarcoma is synonymous with ALT and is generally reserved to cases that occur in intraabdominal, retroperitoneal, mediastinal, or paratesticular regions due to increased morbidity and increased risk of dedifferentiation in these sites). In general, ALT is relatively more cellular with more pleomorphic adipocytes. SCL can have multivacuolated lipocytes with nuclei that are compressed to the periphery, called pseudolipoblasts but will lack true lipoblasts seen in ALT. SCL are typically smaller in size (5 cm in average) than ALT which can be very large.
Although not required for diagnosis, SCL have shown consistent rearrangements of 16q and 13q in cytogenetic studies, specifically deletion of 13q14 region containing the RB1 gene (retinoblastoma protein)[19]. Loss of RB expression by immunohistochemistry has also shown potential diagnostic utility[19]. Also, SCL should not have amplification of MDM2 or CDK4 on analysis by FISH that is associated with ALT.
From a pathologic stand point, surgical excision is generally recommended for both SCL and ALT, the top entity in the differential, as this is usually curative when negative margins are achieved. Both also tend to arise in sites that are amenable to surgical excision (extremities or subcutaneous fat of posterior neck, upper back, and shoulder girdle). Anatomic site of occurrence is the most important prognostic factor for ALT/WDL[20]. Conservative management could be a practical option for either SCL or ALT in a favorable site (low risk of dedifferentiation) given that both would be slow growing and have zero chance of metastasis.
Management of most cases of spindle cell lipoma in the literature was excisional biopsy for undisclosed reasons. Poor ultrasound visualization or concern for well-differentiated liposarcoma after core needle biopsy were cited as the reason for pursuing excisional biopsy in some case reports. In the case of the 28 year old woman with spindle cell lipoma, there was no evidence of tumor recurrence 12 months postoperatively despite incomplete surgical resection[5]. The outcome of that case published in 1999 supported the benign nature of spindle cell lipomas and justifies conservative approach to management. Although our breast surgeon also decided on conservative management for our patient, the ideal management of these lesions remains unclear given the paucity of spindle cell lipoma literature[10]. Given the favorable site of SCL within the breast in the patient in our case and that it was a nonpalpable, incidental finding on a screening mammogram, conservative management with routine annual mammograms and clinical examination was selected.
References
- 1. Enzinger, F.M., Harvey, D.A. Spindle cell lipoma. (1975) Cancer 36(5): 1852–1859.
PubMed||CrossRef||Others
- 2. Chandrashekhar, P., Jose, M., Dadhich, M., et al. Spindle cell lipoma: a case report and review of literature. (2012) Kathmandu Univ Med J (KUMJ) 10(2): 92–95.
- 3. Domanski, H.A., Carlén, B., Jonsson, K., et al. Distinct cytologic features of spindle cell lipoma. A cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. (2001) Cancer 93(6): 381–389.
- 4. Lew, W.Y. Spindle cell lipoma of the breast: A case report and literature review. (1993) Diagn Cytopathol 9: 434-437.
- 5. Mulvany, N.J., Silvester, A.C., Collins, J.P. Spindle cell lipoma of the breast. (1999) Pathology 31(3): 288-291.
PubMed||CrossRef||Others
- 6. Jaffar, R., Zaheer, S., Vasenwala, S.M., et al. Spindle cell lipoma breast. (2008) Indian J Pathol Microbiol 51(2): 234-236.
- 7. López-Ríos, F., Alberti, N., Pérez-Barrios, A., et al. Aspiration Biopsy of Pleomorphic Lipoma of the Breast. (2000) Acta Cytologica 44(2): 255-258.
- 8. Smith, D.N., Denison, C.M., Lester, S.C. Spindle Cell Lipoma of the Breast: A Case Report. (1996) Acta Radiol 37(6): 893-895.
- 9. Howayda, S. Abd El All. Breast spindle cell tumours: about eight cases. (2006) Diagn Pathol 1: 13.
PubMed||CrossRef||Others
- 10. Chan, K.W., Ghadially, F.N., Alagaratnam, T.T. Benign spindle cell tumour of breast: a variant of spindled cell lipoma or fibroma of breast? (1984) Pathology 16(3): 331–336.
- 11. Karakas, C. Spindle cell lipoma / pleomorphic lipoma. (2017) PathologyOutlines.com
PubMed||CrossRef||Others
- 12. Medeiros, M.M., Graziano, L., Alves de Souza, J., et al. Hyperechoic breast lesions: anatomopathological correlation and differential sonographic diagnosis. (2016) Radiol Bras 49(1): 43–48.
PubMed||CrossRef||Others
- 13. Linda, A., Zuiani, C., Lorenzon, M., et al. The wide spectrum of hyperechoic lesions of the breast. (2011) Clin Radiol 66(6): 559–565.
- 14. Linda, A., Zuiani, C., Lorenzon, M., et al. Hyperechoic lesions of the breast: not always benign. (2011) AJR Am J Roentgenol 196: 1219–1224.
PubMed||CrossRef||Others
- 15. Roveda, D., Jr, Piato, S., Oliveira, V.M., et al. Predictive values of BIRADS categories 3, 4 and 5 in non-palpable breast masses evaluated by mammography, ultrasound and magnetic resonance imaging. (2007) Radiol Bras 40: 93–98.
PubMed||CrossRef||Others
- 16. Pardal, R.C., Abrantes, A.F.L., Ribeiro, L.P.V., et al. Screening of breast lesions: a comparative study between mammography, B-mode ultrasonography, sonoelastography and histological results. (2013) Radiol Bras 46: 214–220.
- 17. Taliaferro, A.S., Fein-Zachary, V., Venkataraman, S., et al. Women’s Imaging: Review Imaging Features of Spindle Cell Breast Lesions. (2017) Am J Roentgenol 2(209): 454-464.
PubMed||CrossRef||Others
- 18. Wood, L., Fountaine, T.J., Rosamilia, L., et al. Cuteneous CD34+ Spindle Cell Neoplasms: Histopathologic Features distinguish Spindle Cell Lipoma, Solitary Fibrous Tumor, and Dermatofibrosarcoma Protuberans. (2010) Am J Dermatopathol 32(8): 764-768
- 19. Chen, B.J., Mariño-Enríquez, A., Fletcher, C.D., et al. Loss of retinoblastoma protein expression in spindle cell/pleomorphic lipomas and cytogenetically related tumors: an immunohistochemical study with diagnostic implications. (2012) Am J Surg Pathol 36(8): 1119-1128.
- 20. Lindberg, M.R. Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma. (2018)
PubMed||CrossRef||Others