Study on the efficiency of the growth of the Pacific Oyster “Crassostrea gigas” in Costa Rica (Ostreoida: Ostreidae) according to mesh types
Fabiola V. Arce, Carlos R. Perez*
Affiliation
Nautical fishing Center (NNP), NationalLearningInstitute (INA), Puntarenas, Costa Rica.
Corresponding Author
Carlos, P.R. Nautical fishing Center (NNP), NationalLearningInstitute (INA), Puntarenas, Costa Rica; Email: cperezreyes@ina.ac.cr
Citation
Carlos, P.R., et al. Study on the Efficiency of the Growth of the Pacific Oyster “CrassostreaGigas” in Costa Rica (Ostreoida: Ostreidae) According to Mesh Types. (2020) J Marine Biol Aquacult 6(1): 13-20.
Copy rights
© 2020 Rongxiang, T. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Oyster culture Costa Rica; Crassostreagigas; Long-line culture; Oyster growth efficiency; Mesh type; Nicoya Gulf.
Abstract
Introduction: The Pacific oyster, Crassostreagigas, is one of the most important mariculture economic activities around the world. Two of the main reasons why it has become such an important aquaculture are, it`s huge range of tolerance to environmental conditions, and low mortality rate in comparison with other cultivations. Based on statistics, this oyster market is stable. In Costa Rica, as in other countries, the over exploitation of marine resources by fisheries has caused the search for other alternatives of survival. This is how the initiative to grow oysters in the Gulf of Nicoya begun under a project managed by the National University of Costa Rica located in Puntarenas. The National Institute of learning (INA) gave technical support to them and JICA offered volunteer expertise to enable the development of this investigation.
Objective: The objective of this study was to differentiate growth according to different sizes of plastic mesh with respect to the use of saran, to improve cultivation techniques in oyster farms.
Methods: The survey was conducted from May to November 2017. The test was performed on long lines with 16 plastic mesh bags of three different diameter sizes, which were placed in suspended lanterns: 3 mm, 6 mm, and 20 mm. The controlused was saran and the study involved 12 plastic mesh bags and 4 control bags. The sample size was 30 specimens perbag for a total of 480 oysters whose average initial dimension was 2.88 mm.
Results: For all of the different mesh sizes tested, there were significant differences with respect to the control which was the saran (t student, T <0.05). When comparing the growth in cm3 of the samples, with respect to the diameter of the meshwork, it was determined that there were significant differences (Kruskall-Wallis = 0.000468, p<0.05). The meshwith a diameter of 20 mm was the most efficient in oyster growth, while saran was the least efficient.
Conclusions: Regarding the increase in oyster meat weight, it can be concluded that bags made of mesh with a larger diameter are more efficient than the use of saran due to t
Introduction
According to the Food and Agriculture Organization of the United Nations - FAO (2019), the phenomenon of aquaculture in the world is a growing production process in recent years, as opposed to catch fishing. This is because in the past there was an unplanned extraction of natural resources which led to overfishing in all the world’s fishing grounds[1,2] mention that the proportion of people involved in fisheries was 83% in 1990 and 67% in 2012, while those engaged in aquaculture increased from 17% to 33% in the same period. For this reason, aquaculture is called the blue revolution as a strategy to generate foreign exchange, employment, and demand satisfaction, detaching the activity from environmental damage[3]. Based on Aguilar[4], from 1998 to 2008 activity grew 4.8 times globally and there is a continuous growth trend.
Within aquaculture activities, the cultivation of the Pacific oyster Crassostrea gigas, is the most cultivated species in the world[5-9]. The reason is derived from its high tolerance to environmental conditions, the potential for rapid growth, and low mortality that allows it to succeed in aquaculture. It is a eurihaline bivalve whose intertidal distribution reaches 40 m in depth. It is an asynchronous and dicogamic oviparous species. It feeds on phytoplankton and seston, which is obtained by filtration through gills, being able to filter 0 to 10 liters of water per hour[10-13]. It can resist the presence of dinoflagellates at sea, demonstrating tolerance to the toxicity that they can give and has therefore been considered as a bioindicator by accumulation of toxins in tissue[14].
The species Crassostrea gigas is native to Japan, although it was artificially introduced to Korea, China, Taiwan, Canada, Mexico and the United States[15-16] mention that oyster production reached a record 4.2 million metric tons per year in 2002. According to Furse, Gallo, Noriega and Ramos, the countries with the highest exportation rates are currently the United States and Japan, although the United Kingdom, Argentina, New Zealand and Brazil are regularly mentioned. Therefore, there is a worldwide-consolidated market for the consumption of this mollusk[17]. Oyster aquaculture is an extensive and intensive practice in coastal lagoons, over time it has become an important source of income for families in coastal areas. In Mexico, for example, 18,991 ton/year are produced, contributing to 37% of national production[18,19] point out that Oyster Crassostrea gigas was the most consumed marine product in Brazil in 2007.
In Costa Rica, the fishing development model was based on the exploitation of natural resources, where the production and consumption behavior eventually caused significant environmental damage that led to environmental and social problems. The Gulf of Nicoya reflects these crises, where traditionally guillemets, lines and ropes were used for fishing[20]. Recently, the crisis continues to deepen due to over exploitation, marine pollution, and the destruction of key habitats such as mangroves and corals in the area[21]. Therefore, the National University of Costa Rica (UNA) and several government institutions such as the National Learning Institute (INA) and recently the State Distance University (UNED), accompany the families of fishermen to new livelihood alternatives through aquaculture. These initiatives began in the 1990s, when the National University of Costa Rica began its first studies of harvesting and production of oyster larvae[22]. According to Pacheco and Ulatethe cultivation of Pacific oysters (Crassostreagigas) occured for the first time in 2001, when a project with the Association of Women of Punta Morales began. From that project, the proposal has now been extended to five points in the Gulf: Punta Cuchillo, Isla Cedros, Isla de Chira, Cerro Gordo and Costa de Pájaros, all using “long line” growing systems with lanterns[23].
From the communities mentioned above, the aqua farm located near Isla Cedros stands out as the area of best harvest results. Isla Cedros, and the Gulf of Nicoya region in general, has particularly good physicochemical water conditions[20]. Therefore, the ACUAMAR farm, located between Isla Cedros and Jesusita, is an ideal place to carry out testing using mesh bags of different diameters. Saran bags are used as a dependent variable since they are never changed during the experiment. Saran was considered since it is extremely popular with farmers today, and the idea of this project is to validate changes through comparison to another type of bag. Thus, this work aims to revise whether there is a difference in growth, in relation to the different sizes of plastic mesh in comparison with the use of saran, to improve the techniques of cultivation in oyster farms.
Materials and Methods
Study Area: The study area is in the Gulf of Nicoya, at coordinates 9.833072 N and -84.886067 W Lambert, between Isla Cedros and Isla Jesusita, Paquera of Puntarenas, Costa Rica. It is an area of rocky reefs with low-energy waters and a muddy sandy bottom at some points. The oyster farm ACUAMAR (Association of Marine Growers of Colorado of Abangares) is located here. This company develops the cultivation of oysters through the “Long line” system, which are sign posted ropes laid along the bottom, where 4-level lanterns made with meshes and lined rods are placed, inside which the oysters are deposited in bags of saran. The number of oysters per mesh depends on the size of the oysters. The lines measure 100 meters long where approximately 60 lanterns are placed. The oysters were supplied by the Mollusks Laboratory of the National University of Costa Rica located in Puntarenas. When they reach the aquafarm, they acclimatize for 10-15 minutes in stored water for 24 hours before being transplanted. Managers have boat access so that they can be able to get to the site. There is also a 24-hour checkpoint to avoid theft or vandalism.
Figure 1: Space location of ACUAMAR farm in the Gulf of Nicoya in Puntarenas, 2017 National Geographic Institute, 2010
Sampling: We collected biological information from May12, 2017 to November 09, 2017. Tests were carried out on the long line lines, 16 plastic mesh bags of three sizes of diameter were placed on the suspended lanterns: 3 mm, 6 mm, and 20 mm. There was a saran control. This resulted in 12 plastic mesh bags and 4 control bags. The sample was 30 specimens per bag for 480 oysters, which had an initial average dimension of 2.88 mm, following the methodology proposed by Acosta, Montes, Cortez, Guevara &Lodeiros[24]. Oysters were placed in bags by crop density according to size, following the table below.
Table 1: Farm oyster density according to size in lanterns
|
Size in(mm) |
Lantern** (0.2 m2/floor) |
|
2-3 |
3000 |
|
4-5 |
2000 |
|
6-9 |
2000 |
|
10-14 |
1000 |
|
15-19 |
1000 |
|
20-24 |
500 |
|
25-29 |
500 |
|
30 |
100 |
|
50-70 |
100 |
|
>70 |
50 |
**Opening 10 mm
National University of Costa Rica, Sidey Arias
Weekly cleans were carried out to avoid obstruction of the mesh by algae and sediment. The same weekly cleans were performed for all treatments. During the cleaning, the oysters were removedand place in a freshwater container. The oysters that were approximately 3 or 4 cm in size were placed in fresh water for 10 minutes, the oysters of 4 cm in size were placed in fresh water for 30 minutes. In addition, to remove parasites and other epibiont organisms from them, a scraping of the oysters was performed with a knife on their top or both sides, to remove the barnacles that had attached.
The unfolding of oysters of 3 or 4 cm were made weekly and oysters at 4 cm, fortnightly. Oyster unfolding was made to order them according to their size at different densities, as shown in Table No. 1, to avoid irregular growth by food competition. In summary, 2,697 measurements were made with the sample units.
Records of physicochemical parameters, such as temperature and oxygen, were taken with a YSI model 55, pH meter with a pH tester 10 and Secchi disc at the following depths: 1.5, 3, 4.5 m and 6 meters; always at 13:00 hours of each observation, as proposed by the authors Gómez, Hernando Campos, & Ramírez and Rodríguez respectively[25,8].
Biometrics
With regard to biometric measurements, the methodology of Ruiz, Vargas, and Calero[35] was used, which consists of taking samples of 30 individuals, where the length, width and thickness were measured all in cm, as well as the shell weight with epibionts (fouling), shell without epibionts (no fouling) and weight of the meat in grams. It should be mentioned that the measurement was made with a caliper type calibrator of 0.05 and the weight with a Roman Ohaus Cs 2000. Subsequently, calculations of the shell volume were performed, and correlations were established between shell and meat, with fouling and without fouling.
Statistics
The data was then analyzed with the Instat Plus (2006) Version 3.033 Statistic program, to establish whether there were significant differences in growth according tomesh size by ANOVA. For this, Kruskall Wallis’ test was applied as a sample with continuous and independent variables, and a test was also carried out to compare between the weight of oyster meat grown in the experimental meshes and to the saran by means of a Mann-Whitney test. Finally, a correlation between the weight and size of the mesh using the correlation coefficient (r) was made. Statistical significance was set to p<0.05.
Ethical, Conflict of Interest and Financial Statements
The authors declare that they have fully complied with all pertinent ethical and legal requirements, both during the study and in the production of the manuscript; that there are no conflicts of interest of any kind; that all financial sources are fully and clearly stated in the acknowledgements section; and that they fully agree with the final edited version of the article. A signed document has been filed in the journal archives.
Results
Biometrics
In all dimensions of the meshes tested, there were significant differences from the control that was the saran (t student, T<0.05). Comparing the growth in cm3 of the others with respect to the diameter of the meshwork, it was determined that there were significant differences (Kruskall – Wallis-0.000468, P<0.05), where the meshwork whose 20 mm diameter was most efficient in the growth of oysters, while the saran was the least efficient. At the third month of harvest, the first oysters with sizes greater than 60 mm recorded, specifically in the 20 mm diameter mesh. At the end of the study, the highest recorded value throughout the study was 83.2 mm in the study.
Table 2: Distribution of T student tests with respect to control and different types of mesh for growth in cm3 of the oyster Crassostreagigas, 2017
|
Meshs type |
Value p |
|
<3 mm |
0,009739642 |
|
3 mm |
0,002279948 |
|
6 mm |
0,016656492 |
|
20 mm |
0,011216436 |
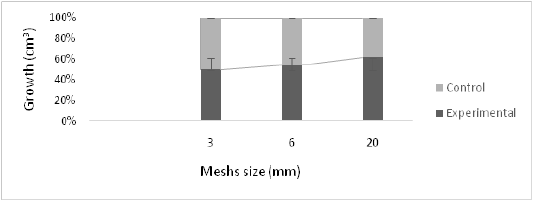
Figure 2: Growth in cm3 recorded in the culture of the oyster Crassostreagigas according to the diameter of the mesh containing it. 2017.
In the 3 mm meshes the average growth was 21.506 ± 20.222 cm3. In the 6 mm ones it was 35 686 ± 23 101 cm3 and in the 20 mm was 42.582 ± 19.574 cm3. Note that the trend is that the larger the size of the meshwork diameter, the greater the growth of the oyster. The largest oyster was 120.238 cm3 recorded in the 20 mm diameter mesh, while the smallest one was recorded in the 3 mm mesh with 763.38 cm3. The largest amount of fouling was recorded in the 20 mm diameter mesh, which required higher operating costs. When the survival of the others between the saran and the different meshes is determined, it is found that there are no significant differences (Test T, p>0.05). Therefore, no matter where they are grown, the survival of the oysters is constant.
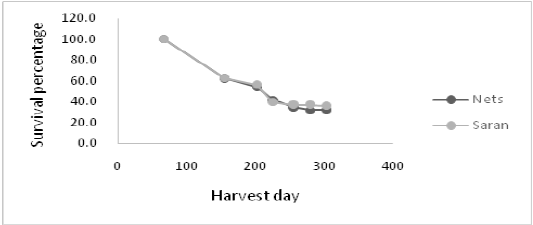
Figure 3: Survival rate of the oysters Crassostreagigas depending on the type of structure of which it is growing, 2017.
With regards to the meat obtained from the experimental meshes, it was determined that there is a direct relationship between the volume in mm3 and the weight of the oyster, considering that the oyster did not contain fouling (Pearson R2= -0.8904, p<0.05). As already mentioned, survival was similar for both control and experimental subjects. However, when comparing the average weight of meat (g) between the saran and the different meshes on average, there were significant differences (Mann-Whitney 0.01804,p<0.05),demonstrating that efficiency is higher in the treatments than in the control. The weight of fouling (g) on average was 0.334 ± 0.582, representing 2% of the total weight of the shelled oyster.
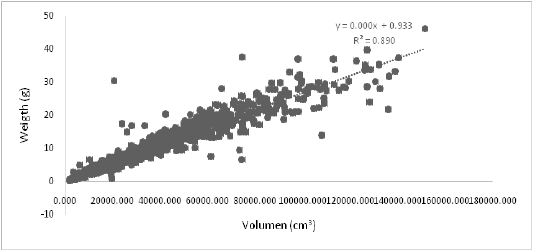
Figure 4: Linear correlation (R2) between the meat weight (g) with respect to the volume of the shell (cm3) on the growth of the Crassostreagigas oyster, 2017.
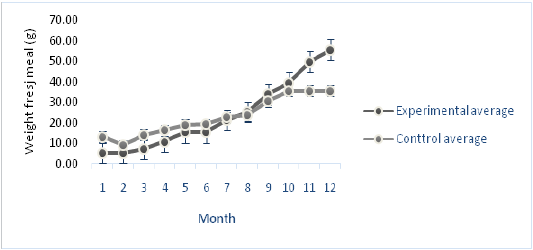
Figure 5: Comparison of the average meat weight (g) of experimental meshes and saran as control
With respect to environmental variables, it was found that the depth (m) on average where the crop was 2,489 ±0.3043 (Pearson R2=-0.0001, p>0.05), the pH was 8.57 ± 0.21 (Pearson R2=- 0.0056, p>0.05), the salinity in ppm was 32,381 ± 1,7909 (Pearson R2= -0.8005, p>0.05), the average temperature was 28.0282 ± 0.70182 (Pearson R2= -0.899, p<0.05) and the BOD in mg L-1 was 7,9757 ± 1.142 (Pearson R2= -0.6429, p>0.05). Linear correlation studies reveal that there is an adverse effect of salinity, temperature, and BOD on meat production by increasing its values. Both light penetration and pH did not reveal that there is any relationship with respect to growth.
Table 3: Chemical physical characteristics of saltwater on average recorded in the cultivation of Crassostreagigas oysters in ACUAMAR, 2017
|
Month |
Secchi (m) |
pH |
Salinity (‰) |
Temperature |
Deep (m) |
|
March |
2.916 |
8.525 |
32.25 |
28.32 |
3.75 |
|
April |
2.636 |
8,54 |
33 |
28.29 |
3.909 |
|
May |
2.75 |
8.575 |
32.75 |
28.23 |
4.25 |
|
June |
2.333 |
8.53 |
33.2 |
27.71 |
4.404 |
|
July |
2.374 |
8.764 |
33.57 |
28.25 |
4.151 |
|
August |
2.077 |
8.3 |
34 |
28.55 |
4 |
|
September |
2.52 |
8.51 |
31.09 |
28.35 |
4.246 |
|
Octuber |
2.05 |
8.55 |
28 |
26.98 |
4.242 |
|
November |
2.75 |
8.5 |
28 |
26.86 |
4.266 |
|
Average |
2.75 |
8.5 |
31.72 |
26.86 |
4.26 |
|
Range[20] |
6-12 |
6-7 |
28-30 |
28-31 |
4-37 |
The effect of seasonality is evident in the table above, where the highest salinity, light penetration and temperature values correspond to the dry season, on the contrary to that in the rainy season. There are significant differences between the dry and rainy season in the physicochemical parameters of water (ANOVA, p<0.05). It is noted that the parameters of light penetration, salinity, pH, and temperature are not in the optimal ranges indicated by the literature with respect to the Gulf of Nicoya.
Discussion
Bags made of meshes of different diameters were more efficient than the use of the saran. Water flow was crucial to this growth, as with the larger mesh work diameters, there was better availability of microalgae for feeding. This shows that, the physiological activities of oysters can be regulated by the design of the aquaculture farm, since it facilitates access to phytoplankton and the removal of organic matter in the water column. This deposition is key to the profitability of the activity[13]. Microalgae is a living food capable of improving aqua farms, even artificial food has not been able to replace its efficiency. The smaller cell size gives better results[8]. In fact, in San Quintín Bay in the United States, despite good demand for oyster production, fluctuations in phytoplankton populations severely affected its supply and the presence of fouling[27,28].
In this work, fouling reached 2% of the average total weight in cultured oysters. It is a negative aspect in the cultivation, since they limit the circulation of water and, therefore, the obtaining of food, as well as the mechanical action of opening and closing the shell. Aguilar also mentions other adverse aspects such as oxygen availability, purification of metabolic residues, damage to buoys, meshes, lanterns and collecting bags that reduce the duration of the productions. The only recommendation given by the author about this inconvenience is the constant replacement of the lanterns. So far, no chemical has worked to eliminate these epibionts, as it negatively affects marine nature.
Another associated risk is the presence of endoparasites and ectoparasites in oysters. This is due to the filtering action, as they lead to concentrated pollutants and parasites such as viruses, bacteria and other organisms[29-32]. Rodríguez et al[8] in their work, indicate that survival can be affected by the presence of epibionts, predation, competition for space and food. An example is the attack of polyquette worms of the genus Boccardiaspp, as an epibiont in crops by increased mortality, the problem is compounded when the oyster reaches a larger size since it has better adhesion surface. This phenomenon can be reduced by decreasing the diameter of the mesh bag, although it leads to a decrease in production in a desired time[34,5].
Based on the above, regulating the diameter of the mesh bags containing the oysters allows to control the production. In a situation where the market is down in terms of demand, the producer can avoid over production of the oyster by changing mesh bag size. Experiences such as that in Baja California between 2006 and 2011, where consumer demand dropped considerably, producers had to decrease production at a high cost[35]. Therefore, if there is a lot of demand the diameter of the mesh should extended, and if there is little demand you can lower it. To clarify,other factors that affect the production of an oyster farm have to do with environmental conditions.
There is an inverse relationship between growth with respect to salinity and temperature because as these increases, oyster growth decreases according to Bartlett and Rosas, Yela and Báez[36,37]. There are parameters that are at the limit or do not reach the optimal level. In this sense, light, pH, salinity, and temperature are not in the optimal range to grow the oyster according to the work of Quesada[20]. Temperature affects the survival of the larva in early stages of harvest, as it appears that thermal stress affects the survival of the larvae and this is also confirmed by Acosta et al in their work. In the case of salinity, Gómez et al[25] warns that high salinity causes a difficulty in the elimination of toxic substances in oysters. While with pH, value well above the limit affects shell production and makes it weak in texture, limiting its survival[38]. Affectation is not only with growth, but also with product quality. Antunes and Ito, Parisenti, et al, Magana, Brito, Gomez y Cruz , Villasenor, et al[39-41] noted in their studies that the temperature, salinity and availability of food affect the amount of fat, proteins, omega 3 and glycogen in oyster meat, something that could be happening in the ACUAMAR area and deserves a more detailed study.
The mortality rate reached 60% in this work, slightly better in the saran than in the experimental meshworks. The rate typically ranged from 40 to 60% of juveniles on Crassotreagigas farms in general. The data obtained from survival and mortality in this work, in line with this range and according to these authors, attribute mortality to water temperature as a determining factor and not so much to the availability of food (phytoplankton). Góngora, García, Hernández and Domínguez-Gómez and Chávez also add the poor management of the farm and natural reasons as other causes as well. Correa, et aland Enríquez[42,15]mention in their studies that high mortality rates can also be due to a homozygous derived from a very small population of breeders and whose founding effect of cultured oysters limits gametic variability, which makes oysters more vulnerable to attack of microorganisms, a fact that forces the farmer to invest in expensive antibiotics to counteract the problem[17]. Regardless of the reasons, the mortality rate for this study was acceptable according to the literature, considering that the highest temperatures occurred in the dry season, which was the time when this harvest began; seasonality is a relevant factor.
This study found variations in growth according to seasonality, of which the causes can be due to several factors. A study by Bautista revealed the presence of bioaccumulated insecticides in tissue especially in the rainy season in Nayarit, Mexico, and results in mortality. The seasonality also of the temperature and the availability of food will become important factors in the cultivation of oysters, this will depend on a good nutritional value. Góngora, Aragon, Dominguez Villanueva[43] demonstrated that Crassostreagigas oysters have seasonal growth patterns in the Gulf of California and do not conform to any proposed mathematical prediction models. In the case of this work, the harvest began in the dry period, characterized by high temperatures and concentrations of chlorophyll according to Vargas, et al[21] and by the experience of the work of Young, et al[13] oysters have better performance in the conditions indicated above.
The first oysters with sexual maturity were obtained in the third month of harvest, based on the claims of Ascencio, Enríquez, Martínez and Aldanaand Rodríguez-Núñez, Toledo, and Arias[44,8] point out that, in fact, the production of gametes starts with sizes greater than 60 mm in length. Therefore, growth was very rapid while the conditions of the dry season remained at a lower but constant rate.
Aquaculture takes place in areas where job opportunities are scarce and represent a source of income for many low-income families[45]. In this sense, the site between Isla Cedros and Jesusita where the ACUAMAR farm is located in the Gulf of Nicoya in Puntarenas, meets the conditions for intensive cultivation, without compromising the capacity of the ecosystem, a factor that Silva, et al[7] considers relevant in productive activity. Not having the influence of industrial complexes and population centers nearby, sewage contamination does not lead to the accumulation of heavy metals or pathogenic microorganisms in tissue of the oyster[46]. Financial and accounting management is essential so that the aqua farm does not have unnecessary expenses and so that production improves[47]. Part of the efficiency consists of improving the production of the aquaculture product, since the costs according to Villanueva are distributed in meshworks, ropes, buoys by 81%, wages by 9% and the rest in process adjustments; costs for new equipment are only in the first year. The chosen site is strategic to making the harvest more efficient. Pereira, Nunes, Nuernberg, Schulz and Vieira[48] claim that a place free of contamination guarantees oysters with high hygienic quality for consumption, free of bacteria and other pathogens.
Despite these tangible benefits of the activity, adverse effects are still under study. One of them is noted in the work of Giberto, et al[49] who describes the danger of the escape of individuals from the oyster to natural environments, since it involves the displacement of native species by the absence of natural predators. Another factor is pointed out by Mann, Burreson and Baker in warning that many of the parasites of these oysters can be transferred to other marine organisms. In view of this, Freitas and Barroso[50] recommend that environmental aspects such as temperature, salinity, BOD and pH, as well as urban development, tourism and fishing in the surroundings of the project, should be framed in a comprehensive management plan of the coastal area, something which should be done the study area, the area where ACUAMAR is it has the support of the community and the support of government organizations, such as the National University of Costa Rica, National Learning Institute and Mix Institute of Social Aid. Activity is growing globally. It is projected that the world’s population will grow from 7 to 9.6 billion people by 2050, with two-thirds of the population living in the area, the first challenge that humanity will face will be to feed the population of the planet, without extinguishing marine resources.
In this sense, aquaculture activity will be the most immediate response to this growing demand for consumption in the future. Projects such as Punta Cuchillowill be considered pioneers in Costa Rica. One recommendation of this study is the combination of aquaculture types. There are works that demonstrate the viability of thisbecause of the condition that it is a biofilter. Pacific oysters can purify the waters from shrimp and tilapia crops in seawater, which are loaded with organic waste of great benefit to them. Studies such as Ramos, et al[51] promote this type of practice, where perhaps salinity can be a limiting but not conditioning factor.
Acknowledgements
We thank the National Learning Institute and International Cooperation Agency of Japan (JICA) for funding this research. Acknowledgments to the National University for having provided the larvae and of course to the Acuamarcorporation for providing us with all the support. A special mention to Mr. Hitochi Natsumoto, who helped us take data in the field and help with the ordering of the data as a JICA volunteer in this project. Thanks to the Techological Management office at INA (Gestión Tecnológica del INA). Thank you to the National Geographic Institute for donating the map of where we performed this work. Thank you to Ericka González and Phoebe Edge from Osa Ecology for the spelling review of the manuscript. Finally, to MARVIVA for support our study.
References
- 1. Ceron, A.N.,Moctezuma, O.R., Monroy, M., et al. Interactive effect of food and water quality on the growth and survival of postlarvae from the river Cambarellusmontezumae. (2015) Rev Mex Bio 86(1): 131-142.
- 2. Gongora-Gomez, A.M., Garcia-Ulloa, M., Hernández-Sepulveda, J.A., et al. Growth of the oyster Crassostreagigas (Thunberg, 1795) cultivated in the La Piedra estuary, Sinaloa, Mexico. (2012) Advances in Agricultural Research, 16(2): 91-104.
PubMed│CrossRef│Others
- 3. Ivanova, A.B., Martha,M.C., Mario,M.S., et al.The blue economy as a model of sustainability for coastal states: the case of Baja California Sur. (2017) Society and Environment 14: 75-98.
- 4. Romulo,E.L. Problems of biofouling in the cultivation of Argopectenpurpuratus in Peru. (2011)RevistaAquatic 35: 9-19.
PubMed│CrossRef│Others
- 5. Gallo-Garcia, M. C., García-Ulloa, M.Boccardia sp. (Polychaete: Spionidae) presence in Crassostreagigas [Thunberg, 1873] oysters reared in the mid coast of the Mexican Pacific. (2005) Advances in Agricultural Research 9(3): 45-48.
PubMed│CrossRef│Others
- 6. Dégremont, L., Edouard, B., Patrick, S., et al. Relative Importance of Family, Site, and Field Placement Timing on Survival, Growth, and Yield of Hatchery-Produced Pacific Oyster Spat (Crassostreagigas). (2005) Aquaculture 249(1-4): 213-229.
- 7. Silva, C., María, A.B., Eleuterio, Y., et al. Application of indicators and models for an ecosystem approach to fisheries and aquaculture: anchovy fishery and Pacific oyster culture in Chile: case studies. (2012) Latin american journal of aquatic research 40(4): 955-969.
- 8. Rodriguez-Nunez, K., Pedro, T., Sidey, A. Isolation of two species of diatoms with aquaculture potential (Bacillariophyceae) in the Pacific of Costa Rica. (2016) UNED Research Notebooks 8(1): 93-98.
- 9. Garcia-Lagunas, N., Romero-Geraldo, R., Kao-Godinez, A.,et al. Differential expression of immune response genes in Pacific oyster, Crassostreagigas spat, fed with dinoflagellates Gymnodiniumcatenatum and Prorocentrum lima. (2019) Latin american journal of aquatic research 47(4): 699-705.
- 10. Mann, R., Burreson, E.M., Baker, P.K. The Decline of the Virginia Oyster Fishery in Chesapeake Bay: Considerations for Introduction of a Non-Endemic Species, Crassostreagigas (Thunberg, 1793). (1991) Journal of Shellfish Research10(2): 379-388.
PubMed│CrossRef│Others
- 11. Furse, Kenmeshh, Jekarina,Gallo.,et al. Cultivation and commercialization of oysters of the Crassostreagigas type. (2004) Lima Peru University of San Ignacio de Loyola.
PubMed│CrossRef│Others
- 12. Villanueva, F.B., Andres, M.G., Norma, P.M.,et al. Growth and Economic Performance of Diploid and Triploid Pacific Oysters Crassostreagigas Cultivated in Three Lagoons of the Gulf of California. (2017)Latin American Journal of Aquatic Research45(2): 466-480.
- 13. Young-Jae, L., Eunah, H., Michael, J.W., et al. Physiological Processes and Gross Energy Budget of the Submerged Longline-Cultured Pacific Oyster Crassostreagigas in a Temperate Bay of Korea. (2018) PLoS One13(7): e0199752.
- 14. Paez-Osuna, F., Cristina, O.M.Biomonitors of coastal pollution with reference to the Mexican coasts: a review on the organisms used. (2011) Hydrobiological 21(3): 229-238.
PubMed│CrossRef│Others
- 15. Correa, F., Collins, E., Oceguera, A., et al. Allozymic variation of the Japanese oyster Crassostreagigas in Bahía San Quintín, Baja California, Mexico. (2004) Marine Sciences 30(1A): 89-97.
PubMed│CrossRef│Others
- 16. Chavez-Villalba, J. Culture of the oyster Crassostreagigas: Analysis of 40 years of activities in Mexico. (2014) Hydrobiological 24(3): 175-190.
PubMed│CrossRef│Others
- 17. Campa-Cordova, A., Antonio, L.G., Jose Manuel, M.S., et al. Effect of probiotic bacteria on the larval culture of the pleasure oyster Crassostreacorteziensis (Bivalvia: Ostreidae). (2011) Journal of Tropical Biology 59(1): 183-191.
PubMed│CrossRef│Others
- 18. Espinoza-Tenorio, A., José Alberto, Z.D., Juan Carlos, N.G., et al. From intuition to scientific knowledge? Publications on the coastal lagoons of Tabasco, Mexico. (2015) Interciencia 40(7): 448-456.
PubMed│CrossRef│Others
- 19. Parisenti, J., Vera Lucia, C.G., Daniel, B.A. Composition of sterols and fatty acids from oysters (Crassostreagigas) grown in Florianópolis - SC, in two seasons. (2010) Food Science and Technology 30(1): 73-76.
- 20. Quesada, R. Identification of the optimal sites for oyster farming in the Gulf of Nicoya, Costa Rica using geographic information systems as input for marine spatial planning (Master’s thesis). (2018) National University, Heredia, Costa Rica.
PubMed│CrossRef│Others
- 21. Vargas-Zamora, J. A., Lopez-Sanchez, M. I., Ramírez-Coghi, A.R. Fishes of the Gulf of Nicoya, Pacific, Costa Rica: update of the lists of the scientific vessels Skimmer and Victor Hensen. (2019) Journal of Tropical Biology 67(4): 913-934.
PubMed│CrossRef│Others
- 22. Rojas-Alfaro, R., Carolina, S.B., Vega-Corrales, L. Biotechnological advances on mariculture in Costa Rica. A review of the research developed by the School of Biological Sciences of the National University. (2017) Unicience 31(2): 111-119.
- 23. Quesada,C.R., Sidey,A.V., Oscar,P.U., et al. Challenges of coastal marine aquaculture: Case of oyster farming in the Gulf of Nicoya, Costa Rica. (2019) in Proceedings of the I International Congress of Exact and Natural Sciences. National University.
- 24. Acosta, V., Marbelis, M., Roraysi, C., et al. Growth of the green mussel Pernaviridis (Bivalvia: Mytilidae) under bottom culture system in the Turpialito inlet, Gulf of Cariaco, Venezuela. (2012) Journal of Tropical Biology 60(4): 1749-1762.
- 25. Gómez, L., Hernando, N., Ramírez, G. Accumulation and purification of aldrin in the oyster Crassostrearhizophorae from the Cienaga Grande de Santa Marta (Colombian Caribbean). (1995) Journal of Tropical Biology 43(1-3): 161-172.
PubMed│CrossRef│Others
- 26. Ruiz, C.A., Erick, U.V., Gerardo, Z.C Production of oyster seed (Crassostreagigas). (2012) UTN Informa 61: 69-73.
PubMed│CrossRef│Others
- 27. García-Esquivel, Z., Marco, A.G., Francisco, L.L., et al. Oyster culture potential in the west arm of San Quintín Bay: Current biomass and preliminary estimate of the carrying capacity. (2004) Ciencias Marinas30(1): 61-74.
PubMed│CrossRef│Others
- 28. Montes,H., Martin, A., Saul, A.B. Impact of two decades of oyster farming on the biomass, abundance and phytoplankton productivity of a coastal lagoon influenced by upwelling. (2007) Hydrobiological 17(3): 213-24.
PubMed│CrossRef│Others
- 29. Gonzalez, A., Marcelo, P.Z., Mariel, C.B., et al. Cell abnormalities in Japanese oyster (Crassostreagigas) larvae from culture. (1999) Marine Research 27: 111-114.
- 30. Sabry, R. C., Magalhaes, A.R. Parasites in cultivated oysters (Crassostrearhizophorae and Crassostreagigas) from Ponta do Sambaqui, Florianópolis, S.C.(2005) Brazilian Archives of Veterinary Medicine and Animal Science 57(suppl 2): 194-203.
- 31. Ramos, R.J., Murilo, A.P., Leticia, A.M., et al. Ocurrence of Vibrio Spp., Positive Coagulase Staphylococci and Enteric Bacteria in Oysters (Crassostreagigas) harvested in the South Bay of Santa Catarina Island, Brazil. (2012) Food Science and Technology 32(3): 478-484.
- 32. Ramos, R.J., Leticia, A.M., Marilia, M., et al.Occurrence of Potentially Pathogenic Vibrio in Oysters (Crassostreagigas) and Waters from Bivalve Mollusk Cultivations in the South Bay of Santa Catarina. (2014)RevSoc BrasMed Trop47(3): 327-333.
- 33. Grijalva, C.J., Manuel, R.L., Tania Lizbeth,E.E., et al. Molecular Evidence of the Protozoan Parasite Marteiliarefringens in Crassostreagigas and Crassostreacorteziensis from the Gulf of California. (2015) Latin American Journal of Aquatic Research43(4): 776-780.
- 34. Gallo-Garcia, M. C., Garcia-Ulloa, A., Rejon-Avina, D. E. et al. Infestation of boring spionids in the oyster Crassostreagigas cultivated in the Laguna de Barra de Navidad, Jalisco, Mexico. (2007) Advances in Agricultural Research 11(3): 63-73.
PubMed│CrossRef│Others
- 35. Cesena, M.A., Luis Carlos, A.B. Analysis of the supply of Japanese oysters in the state of Baja California Sur (Mexico). (2016) University & Business 18(30): 75-96.
- 36. Bartlett, B.R. Biochemical Changes in the Pacific Oyster, Crassostreagigas (Thumberg, 17951, During Larval Develoment and Metamorphosis. (1979) UMI Dissertation Services.
PubMed│CrossRef│Others
- 37. Rosas, I., Alma, Y., Armando, B. Bacteria indicating fecal contamination in oysters (Crassostreavirginica) during their development and processing in the market. (1985) International Journal of Environmental Pollution 1(1): 51-64.
PubMed│CrossRef│Others
- 38. Flores-Higuera, F., Luis-Villasenor, I.E., Rochin-Arenas, J.A.,et al. Effect of pH on the bacterial community present in larvae and spat of Crassostreagigas. (2019)Latin american journal of aquatic research47(3): 513-523.
- 39. Antunes, S. A., Yasuzo, Ito. Chemical Composition of Oysters from Sao Paulo and Parana, Brazil. (1968) Oceanographic Institute Bulletin17(1): 71-88.
- 40. Magana-Carrasco, A., Brito-Manzano, N., Gomez-Vazquez, A., et al.Efects of Temperature and Salinity on Inducing Spawning in the Eastern Oyster (Crassostreavirginica) under Laboratory Conditions. (2018) Ecosystems and agricultural resources5(14): 239-246.
- 41. Villasenor, L., Zamudio-Armenta, O.O., Domenico,V.,et al. Bacterial communities of the oysters crassostreacorteziensis and c. sikamea of cospita bay, sinaloa, mexico. (2018)
International Journal of Environmental Pollution34(2): 203-213. - 42. Enriquez-Espinoza, T., Grijalva-Chon, J.M.Genetic Variability of Crassostreagigas and Crassostreacorteziensis from a Hatchery in Northwestern Mexico. (2010) Ciencias Marinas 36(4): 333-344.
- 43. Gongora-Gomez, A.M., Aragon-Noriega, E.A., Dominguez-Orozco, A.L.,et al. Modeling the Individual Growth of the Pacific Oyster Crassostreagigas Cultivated in the Gulf of California Using the von Bertalanffy Model. (2017) Journal of Marine Biology and Oceanography52(1): 181-185.
- 44. Ascencio, L.A., Martha, E., Imelda, M.M., et al. Effect of temperature and salinity on the reproductive cycle of Crassostreavirginica (Bivalvia: Ostreidae) females and males. (2016) Journal of Tropical Biology 64(2): 449-459.
- 45. Pereira, T.D., Antonio, C.C., Helen Roberta, S.F., et al. Shellfish extractivism in the island of maranhão (ma): ecological and socioeconomic implications. (2017) Public Policy Review 21(2): 831-853.
- 46. Gongora-Gomez, A.M., Manuel, G.U., Norma Patricia, M.S., et al. Heavy-Metal Contents in Oysters (Crassostreagigas) Cultivated on the Southeastern Coast of the Gulf of California, Mexico. (2017) Hydrobiology 27(2):219-227.
- 47. Nascimento, C.D., Alessandra, V.G. Accounting as an Information Basis for the Development of Aquaculture: A Multi-Case Study. (2008) Management &Regionality 24(69): 35-45.
PubMed│CrossRef│Others
- 48. Pereira, M.A., Nunes, M.M., Leonardo, N., et al. Microbiological Quality of Oysters (Crassostreagigas) Produced and Commercialized in the Coastal Region of Florianópolis - Brazil. (2006) Brazilian Journal of Microbiology37(2): 159-163.
- 49. Giberto, D.A., Claudia, S.B., Laura, S., et al. The pacific oyster Crassostreagigas (thunberg, 1793) in the province of buenosaires: natural recruitments in bahiasamborombon. (2012) Fisheries Research and Development Magazine 21: 21-30.
PubMed│CrossRef│Others
- 50. Freitas, R.R., Gilberto, F.B. Conflicts in the use of coastal resources: challenges for the sustainability of mollusc farming. (2006)Virtual Tourism Notebook 6(2): 43-50.
PubMed│CrossRef│Others
- 51.Ramos, R., Luis, V., Walter, S., et al. Treatment of Shrimp Effluent by Sedimentation and Oyster filtration using Crassostreagigas and C. rhizophorae. (2009) Brazilian Archives of Biology and Technology52(3): 775-783.












