Targeting Tumor-Associated Macrophages (TAMs) Reprogramming for Cancer Metastasis Therapy
Affiliation
Department of Clinical Pharmacy, People’s Hospital of Yan’an, China
Corresponding Author
Dr. Haipeng Dong, Department of Clinical Pharmacy, People’s Hospital of Yan’an, China; Email: tingdong@mit.edu
Citation
Dong, H. Targeting Tumor-Associated Macrophages (Tams) Reprogramming for Cancer Metastasis Therapy. (2019) J Pharma Pharmaceutics 6(1): 35- 39.
Copy rights
© 2019 Dong, H. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Tumor-associated macrophages (TAM); Tumor microenvironment (TEM); Tumor metastasis
Abstract
Tumor-Associated Macrophages (TAMs) are critical components of the microenvironment of the majority of malignant tumors, associated with poor prognostic notably through various factors secreting. Thus they are emerging as novel targets for tumor metastasis therapy. In this review article, we describe how TAMs regulate tumor angiogenesis, invasion, metastasis, and then discuss the potential of applying TAMs-targeting treatment as a promising therapeutic strategy for metastatic cancer.
Introduction
Metastasis defined as the spreading of cancer cells from the primary tumor to surrounding tissues and distant organs, it is a foremost event leading to approximately 90% mortality of patients with cancer[1-3]. Despite the advent of effective cancer therapies by developing early diagnosis or applying cancer growth inhibition strategy in the past decades, conventional strategies of cancer therapy include surgical resection, chemotherapy, radiotherapy and immunotherapy, which have made significant contributions to cancer treatment. Limited success has been made in the treatment of metastasis owing to its systemic nature and the resistance of disseminated tumor cells to existing therapeutic agents. Metastasis suppression is still a crucial step for the success of cancer therapy[4,5]. On the basis of evidence from a growing body of research indicating tumor associated macrophages are crucial to cancer metastasis, we summarized the information that is currently at hand and discus the potential therapeutic strategies used to suppress metastatic process, our review highlights the combination therapeutic options to treat cancer metastasis. Because the cellular and molecular programs that drive cancer metastasis. Although our understanding of cellular and molecular programs that drive cancer metastasis remains quite incomplete. Thus, we here we summarized the information that is currently at hand and aiming to expecting a more efficient therapy strategy.
Tumor micro environment (TEM) and metastasis: Tumor metastasis usually goes through a series of sequential and interrelated steps that can be conceptualized as the invasion-metastasis cascade. Starting with a detachment of metastatic cells from the primary tumor, traveling to the surrounding sites or organs intravasation of these cells into the circulatory system and survival, arrest and extravasation through vascular walls into the parenchyma of distant tissues; formation of micro metastatic colonies in this parenchyma; and the subsequent proliferation of microscopic colonies into overt, clinically detectable metastatic lesions, this last process beingtermed colonization[6-9]. Tumor Micro Environment (TME) is intimately involved in all essential steps of the metastasis process through interacting with the tumor. Recently, increasing evidence shows that TME participates aberrant tissue function and promote the subsequent evolution of more stubborn and advanced malignancies.
In general, TME mainly consists of genetically heterogeneous cancer cells, endothelial cells, cancer-associated fibroblasts(CAFs), and different populations of immune cells[2], establishing a complex cross-talk with tumor via producing growth factors, chemokines and matrix-degrading enzymes. For example, CAFs secrete PDGF, FAP, FGFR and VDR, which are participating in wound healing, Integrating collagen and protein to form the ECM fiber network or escaping damage; Immune cells produce TNF-α, IL-10, IL-12, TGF-β and HMGB1, which are not only treat wound healing, infection, clear dead cells and cellular debris but also promote cancer cell proliferating, showing a double effect on tumor formation[10-12].
CAFs are the dominant cell type in the tumor stroma, which exhibits mesenchymal-like features and are likely mesoderm-derived. They are recruited and activated by cancer cells. The interplay between CAFs and cancer cells within the TME is complicated, resulting in various impact on cancer progression and metastasis[13-17]. Large amounts of work described pro-tumorigenic influence of CAFs on cancer cells driven by altered secretome, such as, CXCL12, CCL7, TGF βs, FGFs, HGF, periostin (POSTN) and TN-C, these secreted factors enhance tumor progression by promoting the survival, proliferation, stemness, and the metastasis-initiating capacity of cancer cells, ultimately assisting cancer metastasis[18-20].
Besides CAFs, immune cells also exhibit crucial role in TEM, broad and comprehensive understanding of immune cells will primarily promote the cancer metastasis study. Among these immune cells, Tumor- Associated Macrophages (TAMs) are one of the most abundant infiltrated in solid tumors, which have been known to orchestrate the TME for tumor invasion and progression and contribute to the metastasis of tumor cells[21-23]. Specifically, TAMs are derived from circulating monocytes and differentiate into M1 or M2 macrophages, gaining specific functional properties within the TEM (shown as in Figure1). Classically activated M1 TAMs suppress cancer progression, while M2 type promotes it. However, the specific phenotype of TAMs depends on the tumor progression stage. In the early stages of tumors progression, TAMs adopt the M1-like phenotype for the inhibition of angiogenesis in conjunction with the activation of tumor immunity. In contrast, TAMs shift to an M2-like state to enhance tumor metastasis by secreting different factors (shown as in Figure1)[24-26]. The most comprehensively described mechanism by which M2 TAMs promote cancer metastasis is to provide factors that enhance metastasis and the establishment of a premalignant niche of malignant cells, the elements are listed as below: Matrix Metallopeptidase 2(MMP2), MMP7, MMP9, epidermal growth factor (EGF), wnt family member 5A(WNT5A), macrophage colony-stimulating factor 1(CSF-1), Semaphorin-4D(Sema 4D), IL-1β, Cathepsin B, TNFα, VEGF,TGF-β[2-3]. Cystatin B (CSTB) and WNT5A stimulate cancer cell migration and invasion; VEGF promotes cancer cell extravasation, and TGF-β stimulates cancer cell proliferation and metastasis through C-Jun and SMAD3 pathway[27-32]. Per these findings, TAMs are therefore emerging as an attractive therapeutic target for the inhibition of tumor growth and cancer metastasis.

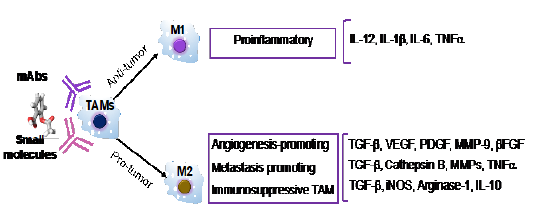
Figure1: The binary M1 / M2 classification of tumor-associated macrophages(TAMs), Pro-tumor M1 type macrophage and anti-tumor M2 type TAM secrete different factors to exact the specific functions.
Targeting TAMs therapeutic strategies: Currently, massive lines of research are being investigated for the effective targeting TAM therapies; the approaches are summarized as the following two routes:
• decreasing the quantity of TAMs in tumor tissue;
• Shifting TAMs from tumor-promoting to tumoricidal status.
A large number of successful attempts have been reported to target TAM via depleting or inhibiting TAMs recruitment[33-38]. The most typical approaches rely on TAM depletion via the inhibition of CSF-1 / CSF-1R or CCL2–CCR2 signaling pathways, based on evidence that these axes are essential for macrophage recruitment[39-41]. Up to now, a variety of small molecules and monoclonal antibodies (mAbs) directed at CSF1R or its ligand CSF1 are investigated for clinical development[34]. Among the class of small molecules, pexidartinib (PLX3397) showed a therapeutic effect in KIT-mutated advanced acral and mucosal melanoma in phase II clinical trial. ARRY-382, PLX7486, BLZ945, and JNJ40346527, are being investigated in solid tumors and cHL via targeting CSF1R[42,43]. MAbs in clinical development include emactuzumab, AMG820, IMC-CS4, cabiralizumab, MCS110, and PD-0360324, with the latter two being the only compounds targeting the ligand CSF1[44-47]. A phase I clinical trial using a CSF-1R–blocking mAb (RG7155) in patients with diffuse-type giant cell tumors (DT-GCT), a proliferative disease caused by overexpression of CSF-1[48], yielded measurable clinical responses. Similarly, the inhibition of CCL2 by an anti-CCL2 monoclonal antibody (e.g., carlumab) or through its synthesis inhibition (e.g., bindarit, trabectedin) prevented the recruitment of macrophages into the tumor site and estimated in various metastatic cancertherapy[49,50]. Besides mAb targeting CCL2, other compounds (e.g., bindarit, trabectedin) were found to inhibit the synthesis of CCL2 / MCP-1. Bindarit reduced TAM and myeloid-derived suppressor cell infiltration in a breast cancer model and resulted in impaired metastatic disease in a prostate cancer model[51]. This treatment also targeted angiogenesis and tumor growth in human melanoma xenografts[52].
In comparison with depleting TAMs, functional reprogramming of TAMs is emerging as a more attractive strategy for cancer metastasis therapy. Bo Yang et al, have proved that Imatinib prevents lung cancer metastasis by interfering the reprogramming of M2-like polarization of macrophages[53]. Enlightenby the relevance of TAMs for metastasis interference scientist’s also sought to re-consider the immune modulatory function of the classical chemotherapeutic drugs. Wanderley CW et al. reported that paclitaxel reduces tumor growth by reprogramming TAMs to an M1 profile in a TLR4-Dependent Manner[54]. Trabectedin, a marine-derived natural product, interferes with transcription and DNA repair but also targets TAMs and induces their depletion through mechanisms as yet obscure[55]. Brana I et al. show that combining Carlumab, a human monoclonal antibody against CCL2, with other chemotherapy agents (docetaxel, gemcitabine, paclitaxel or carboplatin and pegylated liposomal doxorubicin) can significantly delay tumor regrowth following chemotherapy[56].
Despite the above mentioned monotherapies including depleting TAMs and re-educating to an M2 phenotype, complementing and / or synergizing with the conventional anti-cancer treatment such as chemotherapy as well as other cancer-immunotherapy approaches. Floris Dammeijer et al. used CSF-1R kinase inhibitor PLX3397 (pexidartinib) to reduce TAMs amount effectively, and then combine with dendritic cell vaccination synergistically enhanced the survival in mice cancer model[57]. Furthermore, a combination clinical study of PLX3397 and Pembrolizumab to treat advanced melanoma and other solid tumors are undergoing[58,59]. Olson et al. improved therapeutic response by depleting of MHCIIlo TAMs in a preclinical breast cancer model which increased the ability of Taxol to induce apoptosis[60].
With the emerging experimental and clinical studies indicating a strong association between cancer metastasis and increased macrophage infiltration in various cancers, consistent with an unbiased transcriptome analysis, the underlying mechanism behind TAMs modulated cancer metastasis is widely explored and can be summarized as involvement intumor angiogenesis, growth, cell migration and invasion, which was assisted by secreting various chemotactic factors. E.g. Urokinase-Type Plasminogen Activator (uPA), Matrix Metallo Proteinase (MMP) and cathepsins are used to break down the basement membrane and remodel the stromal matrix. Meanwhile, various growth factors and chemokines like Epidermal Growth Factor (EGF), Transforming Growth Factor-Β (TGF-β), Interleukin-8 (IL-8) and Tumor Necrosis Factor-Α (TNF-α) are mostly promoting the migration of tumor cells towards vessels and provide proliferative and anti-apoptotic signals to these cells. Thus, strategies aimed at targeting TAMs for cancer metastasis therapy is gaining the most attention recently. A number of these agents are already currently under clinical investigation. Thus, either monotherapy or in combination with novel and standard cancer therapy strategies are worthwhile to explore.
Acknowledgments: The authors apologize to all others whose work they could not cite because of space restrictions.
References
- 1. Valastyan, S., Weinberg, R.A. Tumor metastasis: molecular insights and evolving paradigms. (2011) Cell 147(2): 275-292.
- 2. Gupta, G.P., Massagué, J. Cancer metastasis: building a framework. (2006) Cell 127(4): 679-695.
- 3. Steeg, P.S. Tumor metastasis: mechanistic insights and clinical challenges. (2006) Nat Med 12(8): 895-905.
- 4. Eccles, S.A., Welch, D.R. Metastasis: recent discoveries and novel treatment strategies. (2007) Lancet 369(9574): 1742-1757.
- 5. Anderson, R.L., Balasas, T., Callaghan, J., et al. A framework for the development of effective anti-metastatic agents. (2018) Nat Rev Clin Oncol 16(3): 185-204.
- 6. Van Zijl, F., Krupitza, G., Mikulits, W. Initial steps of metastasis: cell invasion and endothelial transmigration. (2011) Mutat Res 728(1-2): 23-34.
- 7. Martin, T.A., Ye, L., Sanders, A.J., et al. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. (2013) Landes Bioscience.
Pubmed| Crossref| Others
- 8. Aslakson, C.J., Miller, F.R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. (1992) Cancer Res 52(6): 1399-1405.
- 9. Chambers, A.F., Groom, A.C., MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. (2002) Nat Rev Cancer 2(8): 563-572.
- 10. Quail, D.F., Joyce, J.A. Micro environmental regulation of tumor progression and metastasis. (2013) Nat Med 19(11): 1423-1437.
- 11. Kessenbrock, K., Plaks, V., Werb, Z. Matrix metalloproteinase’s: regulators of the tumor microenvironment. (2010) Cell 141(1): 52-67.
- 12. Joyce, J. A., Pollard, J.W. Microenvironmental regulation of metastasis. (2009) Nat Rev Cancer 9(4): 239-252.
- 13. Bhowmick, N. A., Neilson, E.G., Moses, H. L. Stromal fibroblasts in cancer initiation and progression. (2004) Nature 432(7015): 332-337.
- 14. Kuzet, S. E., Gaggioli, C. Fibroblast activation in cancer: when seed fertilizes soil. (2016) Cell tissue Res 365(3): 607-619.
- 15. Cirri, P., Chiarugi, P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. (2012) Cancer Metastasis Rev 31(1-2): 195-208.
- 16. Räsänen, K., Vaheri, A. Activation of fibroblasts in cancer stroma. (2010) Exp cell Res 316(17): 2713-2722.
- 17. Xouri, G., Christian, S. Origin and function of tumor stroma fibroblasts. (2010) Semin Cell Dev Biol 21(1): 40-46.
- 18. Orimo, A., Gupta, P.B., Sgroi, D.C., et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. (2005) Cell 121(3): 335-348.
- 19. Mezawa, Y., Orimo, A. The roles of tumor- and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. (2016) Cell Tissue Res 365(3): 675-689.
- 20. Raz, Y., Erez, N. An inflammatory vicious cycle: Fibroblasts and immune cell recruitment in cancer. (2013) Exp Cell Res 319(11): 1596-1603.
- 21. Allavena, P., Sica, A., Solinas, G., et al. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. (2008) Crit Rev Oncol Hematol 66(1): 1-9.
- 22. Lewis, C.E., Jeffrey, W.P. Distinct role of macrophages in different tumor microenvironments. (2006) Cancer Res 66(2): 605-612.
- 23. Condeelis, J., Pollard, J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. (2006) Cell 124(2): 263-266.
- 24. Solinas, G., Germano, G., Mantovani, A., et al. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. (2009) J Leukoc Biol 86(5): 1065-1073.
- 25. Schioppa, T., Mantovani, A., Allavena, P., et al. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. (2006) Eur J Cancer 42(6): 717-727.
- 26. Kurahara, H., Shinchi, H., Mataki, Y., et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. (2011) J Surg Res 167(2): e211-e219.
- 27. Lee, G.T., Kang, D.I., Ha, Y.S., et al. Prostate cancer bone metastases acquire resistance to androgen deprivation via WNT5A-mediated BMP-6 induction. (2014) Br J Cancer 110(6): 1634–1644.
- 28. Thomas, A.W., Ajay, C., Jeffrey, W. Macrophage biology in development, homeostasis and disease. (2013) Nature 496(7446): 445–455.
- 29. Sierra, J.R., Corso, S., Caione, L., et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages (2008) J Exp Med 205(7): 1673-1685.
- 30. Nielsen, S.R., Schmid, M.C. Macrophages as Key Drivers of Cancer Progression and Metastasis. (2017) Mediators Inflamm 2017: 9624760.
- 31. Zhang, S., Che, D., Yang, F., et al. Tumor-associated macrophages promote tumor metastasis via the TGF-β/SOX9 axis in non-small cell lung cancer. (2017) Oncotarget 8(59): 99801-99805.
- 32. Noy, R., Pollard, J.W. Tumor-associated macrophages: from mechanisms to therapy. (2014) Immunity 41(1): 49-61.
- 33. Gazzaniga, S., Bravo, A.I., Guglielmotti, A., et al. Targeting Tumor-Associated Macrophages and Inhibition of MCP-1 Reduce Angiogenesis and Tumor Growth in a Human Melanoma Xenograft. (2007) J Invest Dermatol 127(8): 2031-2041.
- 34. Wyckoff, J.B., Wang, Y., Lin, E.Y., et al. Direct Visualization of Macrophage-Assisted Tumor Cell Intravasation in Mammary Tumors. (2007) Cancer Res 67(6): 2649-2656.
- 35. Lin, E.Y., Li, J.F., Gnatovskiy, L., et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. (2006) Cancer Res 66(23): 11238-11246.
- 36. Chen, Q., Zhang, X.H., Massagué, J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. (2011) Cancer Cell 20(4): 538-549.
- 37. Qian, B., Deng, Y., Im, J. H., et al. A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth. (2009) PLoS One 4(8): e6562.
- 38. Ruffell, B., Affara, N.I., Coussens, L.M. Differential macrophage programming in the tumor microenvironment. (2012) Trends Immunol 33(3): 119-126.
- 39. Zheng, X., Turkowski, K., Mora, J., et al. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer therapy. (2017) Oncotarget 8(29): 48436-48452.
- 40. Petty, A.J., Yiping, Y. Tumor-associated macrophages: implications in cancer immunotherapy. (2017) Immunotherapy 9(3): 289-302.
- 41. Cannarile, M.A., Weisser, M., Jacob, W., et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. (2017) J Immunother Cancer 5(1): 53.
- 42. Sreekanth, A. R., Sandeep, K. M., Manvendra, P. S., et al. Design, synthesis and optimization of bis-amide derivatives as CSF1R inhibitors. (2017) Bioorg Med Chem Lett 27(10): 2153-2160.
- 43. Cassier, P. A., Kluźniak, W., Huzarski, T., et al. Clinical outcomes in women with breast cancer and a PALB2 mutation: a prospective cohort analysis. (2015) Lancet Oncol 16(6): 949-956.
- 44. Papadopoulos, K.P., Gluck, L., Martin, L.P., et al. First-in-Human Study of AMG 820, a Monoclonal Anti-Colony-Stimulating Factor 1 Receptor Antibody, in Patients with Advanced Solid Tumors. (2017) Clin Cancer Res 23(19): 5703-5710.
- 45. Peyraud, F., Sophie, C., Antoine, I. CSF-1R Inhibitor Development: Current Clinical Status. (2017) Curr Oncol Rep 19(11): 70.
- 46. Ries, C.H., Hoves, S., Cannarile, M.A., et al. CSF-1/CSF-1R targeting agents in clinical development for cancer therapy. (2015) Curr Opin Pharmacol 23: 45-51.
- 47. Cassier, P.A., Italiano, A., Gomez-Roca, C.A., et al. CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: a dose-escalation and dose-expansion phase 1 study. (2015) Lancet Oncol 16(8): 949-956.
- 48. Pienta, K.J., ., Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. (2013) Invest New Drugs 31(3): 760-768.
- 49. Brana, I. ., ., et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. (2015) Targeted Oncol 10(1): 111-123.
- 50. Zollo, M., Di Dato, V., Spano, D., et al. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. (2012) Clin Exp Metastasis 29(6): 585-601.
- 51. Gazzaniga, S., Bravo, A.I., Guglielmotti, A., et al. Targeting Tumor-Associated Macrophages and Inhibition of MCP-1 Reduce Angiogenesis and Tumor Growth in a Human Melanoma Xenograft. (2007) J Invest Dermatol 127(8): 2031-2041.
- 52. Yao, Z., Jieqiong, Z., Bo, Z., et al. Imatinib prevents lung cancer metastasis by inhibiting M2-like polarization of macrophages. (2018) Pharmacol Res 133: 121-131.
- 53. Wanderley, C.W., Colón, D.F., Luiz, J.P.M., et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. (2018) Cancer Res 78(20): 5891-5900.
- 54. D’incalci, M., Badri, N., Galmarini, C.M., et al. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. (2014) Br J Cancer 111(4): 646-650.
- 55. Brana, I., Calles, A., LoRusso, P.M., et al. Carlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. (2015) Target Oncol 10(1): 111-123.
- 56. Dammeijer, F., Lievense, L.A., Kaijen-Lambers, M.E., et al. Depletion of Tumor-Associated Macrophages with a CSF-1R Kinase Inhibitor Enhances Antitumor Immunity and Survival Induced by DC Immunotherapy. (2017) Cancer Immunol Res 5(7): 535-546.
- 57. Robert, C., Ribas, A., Wolchok, J.D., et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. (2014) Lancet 384(9948): 1109-1117.
- 58. Armand, P., Margaret, A. S., John, K., et al. A phase 2 study of a nivolumab (nivo)-containing regimen in patients (pts) with newly diagnosed classical Hodgkin lymphoma (cHL): Study 205 Cohort D. (2016) J Clin Oncol 34(31): 3733.
Pubmed| Crossref| Others
- 59. Olson, O.C., Kim, H., Quail, D.F., et al. Tumor-Associated Macrophages Suppress the Cytotoxic Activity of Antimitotic Agents. (2017) Cell Reports 19(1): 101-113.












