The Effect of Intravenous Infusion of Magnesium Sulfate during Surgery on Pain Reduction after Caesarean Section with Spinal Anesthesia
Anahita Hirmanpour1, Azim Honarmand2, Elham Naghshineh3, Sahar Eskandari2, Sahar Eskandari4, Habib Jalali4
Affiliation
- 1Assistant Professor of Anesthesia, Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 2Professor of Anesthesia, Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
- 33Assistant Professor of Obstetrics & Genecology, Isfahan University of Medical Sciences, Isfahan, Iran
- 44Isfahan University of Medical Sciences, Isfahan, Iran
Corresponding Author
Mohammadreza Safavi, Professor of Anesthesia, Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran, Tel: 00989133152416, E-mail: safavi@med.mui.ac.ir
Citation
Safavi, M., et al. The Effect of Intravenous Infusion of Magnesium Sulfate during Surgery on Pain Reduction after Caesarean Section with Spinal Anesthesia. (2017) J Anesth Surg 4(1): 15- 22.
Copy rights
© 2017 Safavi, M. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Caesarean section; Postoperative pain; Magnesium sulfate; Spinal anesthesia
Abstract
Background: This study evaluated the effect of infusion of magnesium sulfate during surgery on pain and Parturient morbidity after caesarean section.
Methods: In this randomized controlled double-blind clinical trial, 40 pregnant women ASA I, II, who were candidates for caesarean under spinal anesthesia were allocated into two groups by block random methods; the intervention group received magnesium sulfate with 40 mg/kg as loading dose and infusion of 15 mg/kg/h until the end of the surgery and the placebo group received normal saline with similar volume. Pain intensity was measured and recorded by numerical rating scale (NRS) at the baseline (motor block started to wear off) and 30 minutes, 4, 12 and 24 hours later.
Results: Magnesium sulfate decreased the mean of blood pressure 3 and 5 minutes after spinal anesthesia and 15 minutes into recovery, decreased the mean of pain intensity (p < 0.001) and painkiller consumption and also reduced the interval before toleration of oral liquids for the time after surgery (10 hours and 53 minutes vs. 13 hours and 6 minutes, p < 0.05). Side effects and the Apgar score of neonates at the first and fifth minutes after delivery had no significant difference between both groups (p < 0.05).
Conclusions: Infusion of magnesium sulfate during surgery would decrease pain intensity and postoperative ileus and also would increase the interval before painkiller request by the mother with the least side effects for the mother and the neonate.
Introduction
Pain after caesarean section is the most common and important complain of mothers at most of the medical centers[1]. Postpartum pain is the worst fear of mothers which would stop them from getting back into shape for taking care of and breastfeeding their neonate. Therefore anesthesiologists should try their best to get mothers to their desirable condition as fast as possible[1,2]. Pain intensity after caesarean section depends on many different factors including type and duration of the surgery, the method of anesthesia, the type of consumed anesthetic and patient’s psychological and mental condition[1,3-5].
Different methods for controlling pain after caesarean section have been studied; non-steroidal and opioid anti-inflammatory drugs is the traditional and most common used method. The side effects of these drugs include digestive problems, nausea and vomiting, respiratory distress, constipation and drowsiness[1,2]. The more modern methods like constant infusion of epidural or patient controlled analgesia (PCA), besides requiring trained personnel and special facilities, are expensive methods that sometimes are associated with various side effects[1,2].
Magnesium sulfate has a widespread application in the field of obstetrics and gynecology. It is used for preventing and treating convulsions in preeclampsia and eclampsia. Magnesium sulfate is an N-Methyl-D-Aspartate and its related channels’ receptor antagonist[6-8] and also has a calcium channel blocker effect which in patients with chronic pain works like opioid drugs[4,8-10]. Magnesium sulfate would prevent the secretion of catecholamine from adrenal medulla and peripheral nerves terminals and would also block catecholamine receptors directly[1,11]. Therefore magnesium would cause sympathetic block and would indirectly lead to vasodilatation and consequently decrease blood pressure[5,12-14].
From 1990, the effect of magnesium and opioid consumption on postoperative pain has been evaluated in gynecologicandocular surgeries, arthroscopic surgical procedures and surgical treatment of lumbar spine[3,9,13,15-17]. In this study the effect of intravenous infusion of magnesium sulfate during surgery on reduction of pain intensity, the need for painkillers and postoperative side effects after caesarean section with spinal anesthesia has been evaluated.
Methods
This study was a randomized controlled double-blind clinical trial which was conducted after taking approval from ethics committee of the research council of Isfahan University of Medical sciences from winter 2015 to autumn 2015 at Shahid Beheshti teaching medical center.
ASA I, II pregnant women who were candidates for caesarean and had no history of liver dysfunction, renal dysfunction (GFR < 60 mL/min/1.73 m²) and cardiovascular problems, especially AV blockage, any heart problems or EF < 40%, any type of heart block, cardiac arrhythmia, diagnosed hypertension or pregnancy hypertension, diabetes, neurologic problems or addiction, diagnosed allergy to magnesium sulfate, myopathy and calcium channel blocker consumption were enrolled in the study. Any changes in the type of anesthesia or surgery which led to hysterectomy or urology interventions, severe hemorrhage and need for massive blood transfusion and lingered surgery more than 2 hours caused exclusion from the study.
Patients were allocated into two groups of magnesium sulfate receivers and normal saline receivers by random block method. The intervention group received 40 mg/kg of magnesium sulfate in 100 cc of normal saline 10 minutes before spinal anesthesia and then infusion of 15 mg/kg/h of magnesium sulfate during the surgery. The control group received similar dosage of normal saline.
The routine monitoring of electrocardiogram, pulse oximetry and non-invasive blood pressure was contacted to patients before spinal anesthesia. After receiving 500 cc of Ringer, spinal anesthesia was conducted by the anesthesiologist using a 25 gauge quincke needle at L3 - L4 level with 0.5 cc of Sufentanyl and 0.2 cc of 0.5% hyper-thin Marcaine.
Patients’ vital signs including systolic, diastolic and the mean of blood pressure, heart rate, oxygen saturation and respiratory rate were measured and recorded before the spinal anesthesia, right after the spinal anesthesia, 3, 5, 10 and 15 minutes after anesthesia and then every half an hour until the end of the surgery and every 15 minutes in the recovery. The maximum level of sensory block was checked and recorded 10 minutes after anesthesia using two-sided Pin Prick at the mid-axillary line. Patients’ pain was measured and recorded with numerical rating scale (NRS) from the time motor block started to wear off at the baseline, 30 minutes and 4, 12 and 24 hours after the surgery using modified Bromage scale grade V*. The rating of pain intensity was as “no pain = 0”, “mild pain = 1 - 4”, “moderate pain = 5 - 7”, and “severe pain = 8 - 10” which pains with score of 4 or more required intervention.
* Modified Bromage scale: 1 = complete motor blockade; 2 = almost complete motor blockade: The patient is able to only move the feet; 3 = partial motor blockade: The patient is able to move the knees; 4 = detectable weakness of hip flexion: The patient is able to raise the leg but is unable to keep it raised; 5 = no detectable weakness of hip flexion: The patient is able to keep the leg raised for at least 10 seconds; 6 = no weakness at all: The patient is able to perform partial knee bend while supine).
In case of an NRS ≥ 4, Diclofenac 100 mg suppository was prescribed for the patients and if the pain continued, 50 mg of Pethidinewas injected intramuscularly. After transferring to the ward, patients who received standard treatment with Diclofenac and Pethidine, based on their request and NRS ≥ 4, again received Diclofenac 100 mg suppository and if needed, 50 mg of intramuscular Pethidine. The first request for painkillers at the recovery and the ward and also the additional consumed dosage of Diclofenac and Pethidine were recorded. The serum level of magnesium in all patients was checked and recorded before starting the infusion and right after the end of the infusion. For all the patients the first time to tolerate oral liquids was recorded and compared between them.
Considering a confidence interval of 95%, power test of 0.84 and accuracy of 0.5, the sample size for each group was calculated to be 20. Statistical analysis was conducted using SPSS 21 (Chicago, Illinois, USA). P value less than 0.05 was considered statistically significant. Qualitative variables were analyzed using t test and Chi-square test. Categorical variables were analyzed using Mann-Whitney test. Variance analysis with repeated measures, Newman-Keuls and post hoc student tests were used for hemodynamic variables at different time in both groups.
Results
Forty pregnant women were enrolled in the study and 1 from each group was excluded due inadequate numbness to undergo general anesthesia. Demographic characteristics and the mean duration of surgery and recovery are respectively shown in (table 1 and 2).
Table 1: Frequency distribution and dispersion of demographic characteristics of the participants (for each group separately).
| Variable | Magnesium group | Placebo group | P value |
|---|---|---|---|
| Age (years) | 29.88 ± 6.1 | 36.61 ± 4.2 | 0.683 |
| Gestational age (weeks) | 38.67 ± 0.7 | 38.06 ± 0.9 | 0.053 |
| Height (cm) | 162.78 ± 5.4 | 160.18 ± 7.8 | 0.257 |
| Weight (kg) | 82.33 ± 14.1 | 81.33 ± 15.7 | 0.842 |
| Body Mass Index (BMI) | 31.01 ± 4.9 | 31.49 ± 4.3 | 0.759 |
A p value of less than 0.05 indicated statistical significance.
Table 2: Comparing the mean duration of surgery and recovery between both groups.
| Variable | Magnesium group | Placebo group | P value |
|---|---|---|---|
| Duration of surgery (minutes) | 61.11 ± 12.9 | 60.56 ± 16.88 | P ≥ 0.05 |
| Duration of recovery (minutes) | 74.41 ± 12.36 | 69.44 ± 12.33 | P > 0.05 |
P value of less than 0.05 indicated statistical significance.
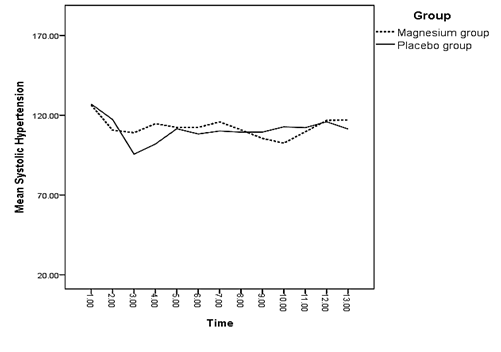
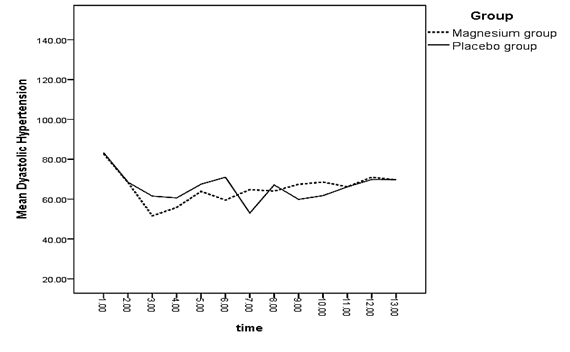
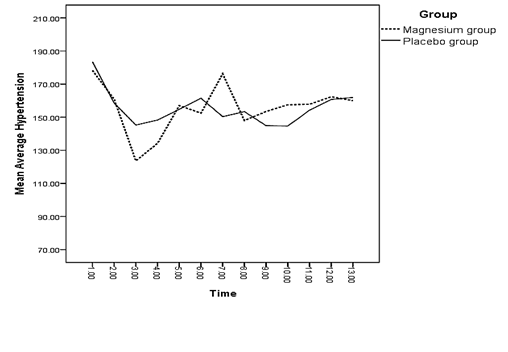
A significant difference was observed between both groups regarding the mean of systolic blood pressure right after and 3 minutes after spinal anesthesia (p < 0.05) (Diagram 1). There was a significant difference between the mean of diastolic blood pressure of both groups 3 and 5 minutes after spinal anesthesia and 15 minutes into recovery (p < 0.05) (Diagram 2).
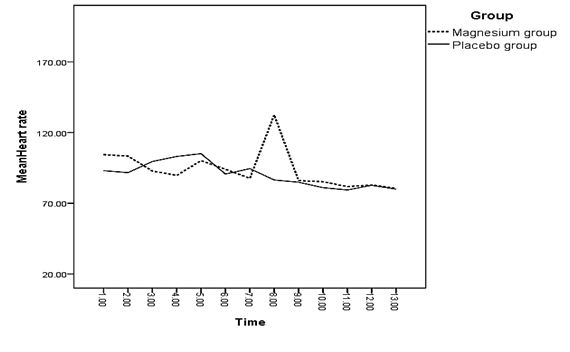
The difference between the mean of heart rate of both groups was only significant right before spinal anesthesia (p = 0.044 < 0.05); meaning that the mean of heart rate in the magnesium receiving group was lower than the placebo group (Diagram 3).
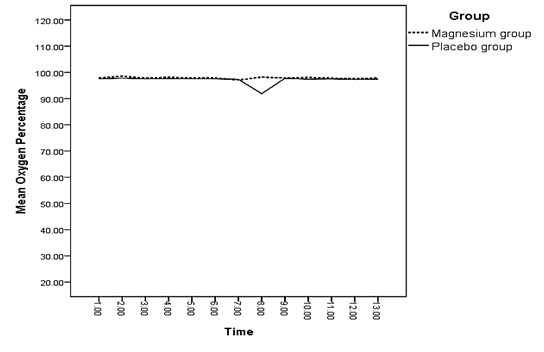
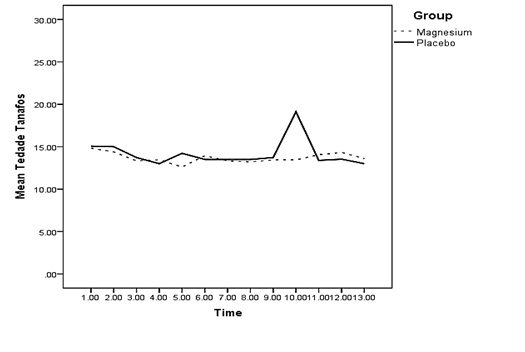
The difference between the mean of changes in oxygen saturation of both groups was not significant at any of the measured times (p > 0.05) (Diagram 4). There was a significant difference between the mean of changes in the mean arterial pressure of both groups 3 and 5 minutes after spinal anesthesia and 15 minutes into recovery (p < 0.05) (Diagram 5). Regarding the mean of respiratory rate, no significant difference was observed between both groups at any of the measured times (p > 0.05) (Diagram 6).
Table 1: Measurement times for diagrams 1 to 6
| Measurement time according to the time of spinal anesthesia | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before anesthesia | Right after | 3 minutes after | 5 minutes after | 10 minutes after | 15 minutes after | 45 minutes after | 75 minutes after | 105 minutes after | 135 minutes after | 165 minutes after | 195 minutes after | 225 minutes after |
Diagram 1:
Diagram 2:
Diagram 3:
Diagram 4:
Diagram 5:
Diagram 6:
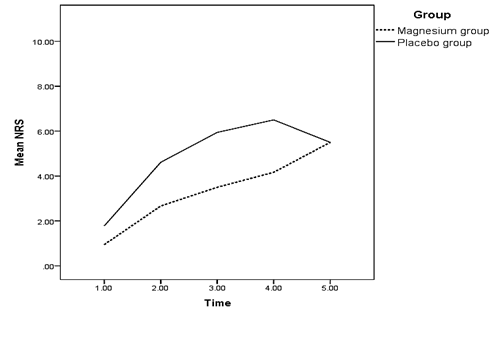
The mean of pain intensity 30 minutes and 4, 12 and 24 hours after motor block wear off had a significant difference between both groups (p < 0.05) (Table 3); in a way that pain intensity in the magnesium receiving group was significantly lower than the placebo group at all the mentioned times. There was no significant difference between the pain intensity of both groups at the time motor block started to wear off (p > 0.05) (Diagram 7).
Table 3: Determining and comparing pain intensity in both groups at different time intervals after surgery according to NRS.
| Time interval | Magnesium group | Placebo group | P value |
|---|---|---|---|
| Start of motor block wear off | 0.94 | 1.78 | 0.185 |
| 30 minutes after motor block started to wear off | 2.67 | 4.61 | 0.014 |
| 4 hours after motor block started to wear off | 3.5 | 5.94 | 0.000 |
| 12 hours after motor block started to wear off | 4.17 | 6.5 | 0.000 |
| 24 hours after motor block started to wear off | 3.44 | 5.5 | 0.000 |
Diagram 7:
In the magnesium receiving group, from the time motor block started to wear off, most of the patients experienced pain with mild intensity and as time passed by, a few percent experienced moderate pain and a fewer percent experienced severe pain. In the placebo group most of the patients experienced mild and moderate pain and, as it can be observed, from the time motor block started to wear off, experiencing pain with severe intensity have increased among the patients of this group. Eventually, 24 hours after motor block wears off, 77.8% of the patients in magnesium group reported mild pain and 66.7% of patients in the placebo group reported moderate pain (Table 4).
| Measurement time according to the time of motor block wear off | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Right after | After 30 minute | After 4 hours | After 12 hours | After 24 hours |
Table 4: Determining and comparing pain intensity in both groups.
| Title | Pain intensity N (%) | Total | |||
|---|---|---|---|---|---|
| Mild | Moderate Severe | Severe | |||
| Start of motor block wear off | Magnesium group | 17 (94.4%) | 1 (5.6%) | 0 (0.0%) | 18 |
| Placebo group | 16 (88.9%) | 2 (11.1%) | 0 (0.0%) | 18 | |
| 30 minutes after motor block wear off | Magnesium group | 16 (88.9%) | 2 (11.1%) | 0 (0.0%) | 18 |
| Placebo group | 7 (39.9%) | 8 (44.4%) | 3 (16.7%) | 18 | |
| 4 hours after motor block wear off | Magnesium group | 14 (78.8%) | 4 (22.2%) | 0 (0.0%) | 18 |
| Placebo group | 6 (33.3%) | 8 (44.4%) | 4 (22.2%) | 18 | |
| 12 hours after motor block wear off | Magnesium group | 11 (61.1%) | 6 (33.3%) | 1 (5.6%) | 18 |
| Placebo group | 0 (0.0%) | 12 (66.7%) | 6 (33.3%) | 18 | |
| 24 hours after motor block wear off | Magnesium group | 14 (77.8%) | 4 (22.2%) | 0 (0.0%) | 18 |
| Placebo group | 5 (27.8%) | 12 (66.7%) | 1 (5.6%) | 18 | |
The difference between the consumed Diclofenac suppositories in both groups during the first 24 hours after motor block wear off was significant (p < 0.05). Regarding the injection of Pethidine, the difference between both groups was only significant during the first 4 hours. It must be noted that in the magnesium receiving group, the highest consumption of Diclofenac and Pethidine happened 12 hours after motor block wear off. Comparison between the frequencies of painkiller consumption in both groups is shown in table 5.
Table 5: Comparing the frequency of painkiller (Diclofenac and Pethidine) consumption in both groups.
| Time interval | Magnesium sulfate group | Placebo group | ||
|---|---|---|---|---|
| Diclofenac suppository | Pethidine | Diclofenac suppository | Pethidine | |
| Start of motor block wear off | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.2%) |
| 30 minutes after motor block wear off | 1 (5.2%)* | 3 (15.7%) | 8 (42.1%) | 3 (15.7%) |
| 4 hours after motor block wear off | 4 (21.05%) | 1 (5.2%) | 13 (68.42%) | 6 (31.57%) |
| 12 hours after motor block wear off | 5 (26.31%) | 3 (15.7%) | 13 (68.42%) | 7 (36.84%) |
| 24 hours after motor block wear off | 3 (15.7%) | 0 (0.0%) | 14 (73.68%) | 1 (5.2%) |
*A p value of less than 0.05 indicated statistical significance.</p >
The frequency of occurrence of side effects had no significant difference between both groups (p > 0.05) (Table 6). 44% of the patients of the placebo group (8 patients) experienced no side effects and the most common side effect in this group was shivering, nausea and vomiting (22%). In the magnesium group 38.9% of patients (7 patients) experienced no side effects; hypotension with the prevalence of 22.2% (4 patients) and then flushing and shivering with the prevalence of 11.1% (2 patients) were the most common side effects.
Table 6: Determining and comparing the frequency of occurrence of side effects after surgery in the both studied groups.
| Title | Frequency | Chi square test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No side effects | Hypotension | Flushing | Nausea and vomiting | Respiratory depression | Arrhythmia | Shivering | Total | P value | ||
| Group | Magnesium | 7 38.9% | 422.2% | 211.1% | 316.7% | 00.0% | 00.0% | 11.1% | 18 | 0.004 |
| Placebo | 8 44.4% | 2 11.1% | 0 0.0% | 4 22.2% | 0 0.0% | 0 0.0% | 4 22.2% | 18 | ||
| Total | 15 | 6 | 2 | 7 | 0 | 0 | 6 | 36 | ||
*A p value of less than 0.05 indicated statistical significance.
Considering a significance level of 5%, Pearson Chi-square test showed no significant difference between the motor block level of both groups (p > 0.05) (Table 7).
Table 7: Determining and comparing the frequency of the level of motor block between both studied groups.
| Title | Frequency of the level of motor block N (%) | Chi square test | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | T3 | T4 | T5 | T6 | T8 | T9 | Total | P value |
| Magnesium | 0 (0.0%) | 11 (61.1%) | 6 (33.3%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | 18 | 0.188 |
| Placebo | 2 (12.5%) | 6 (37.5%) | 5 (31.2%) | 2 (12.5%) | 1 (6.2%) | 0 (0.0%) | 18 | |
| Total | 2 | 17 | 11 | 2 | 1 | 1 | 36 | |
*A p value of less than 0.05 indicated statistical significance.
The mean serum level of magnesium before infusion in both groups was similar and no significant relation between the studied group and the serum level of magnesium was observed (p > 0.05) (Table 8). On the other hand the mean serum level of magnesium after infusion in the magnesium receiving group was higher than the placebo group; the relation between the serum level of magnesium and the magnesium receiving group after infusion was significant (p = 0.000 < 0.05). The serum level after infusion still was in the normal range (Table 8).
Table 8: Determining and comparing the mean serum level of magnesium in both groups.
| Variable | Magnesium sulfate group | Placebo group | P value |
|---|---|---|---|
| Before infusion | 1.61 ± 0.14 | 1.61 ± 0.15 | 0.920 |
| Right after infusion | 2.13 ± 0.33 | 1.66 ± 0.14 | 0.000 |
*A p value of less than 0.05 indicated statistical significance.
Data are presented as mean ± SD
There was a significant difference between the mean time of oral administration in both groups (p = 0.015 < 0.05); in a way that the mean time interval before tolerating oral liquids in the magnesium receiving group was 10 hours and 53 minutes and in the placebo group was 12 hours and 6 minutes (Table 9).
Table 9: Determining and comparing the mean of the first time to tolerate oral liquids in both groups.
| Title | Mean | Standard deviation | Pearson chi-square test | 95% confidence interval | |||
|---|---|---|---|---|---|---|---|
| Value | P Value | Upper | Lower | ||||
| Group | Magnesium | 10.53 | 2.58 | 2.569 | 0.015 | -0.441 | -3.848 |
| Placebo | 12.66 | 2.21 | |||||
*A p value of less than 0.05 indicated statistical significance.
The mean of Apgar score of neonates 1 and 5 minutes after delivery had no significant difference between both groups (Table 10) (p > 0.05).
Table 10: Determining and comparing the mean of Apgar score of neonates in both groups.
| Variable | Magnesium group | Placebo group | P value |
|---|---|---|---|
| Apgar score at The first minute | 8.94 | 9 | 0.324 |
| Apgar score at the fifth minute | 10 | 10 | 0.331 |
*A p value of less than 0.05 indicated statistical significance.
Discussion
Pain control after surgery and reducing its related morbidity, would decrease the duration of patients’ hospitalization and treatment expenses, which, especially in developing countries, is of great importance[5]. The main result of this study was that prescribing magnesium sulfate during caesarean section with spinal anesthesia, along with hemodynamic stability of patient during surgery, could significantly decrease patient’s pain and consumption of non-steroidal anti-inflammatory drugs and opioid, in addition could also decrease the period of ileus after caesarean section; this could have an important in fast discharge of the patient from the hospital. Faster return of the mother to the family has an especial importance in the mental health and hygiene of the family and the society. Considering a meta-analysis that was conducted in 2013, the palliative effect of magnesium sulfate has been approved by a few studies and further studies are required in this field[10].
The definite lingered analgesic effect of magnesium sulfate could be caused by inhibiting the receptors of N-Methyl-D-Aspartate (NMDA)[6,10]. Prescribing intravenous magnesium sulfate with bolus dose in back surgery has decreased the consumption of painkillers after surgery and has also led to better sleeping and more satisfaction during the first 24 hours after surgery in patients[1]. The present study also revealed decreased consumption of painkillers and increased satisfaction in patients which a suggested mechanism for this matter is magnesium sulfate’s effect as “the physiologic blocker of calcium channel”[18]. This analgesic effect in knee arthroscopy for patients who received bolus and infusion of magnesium sulfate significantly decreased the consumption of fentanyl during and after surgery for 4 hours after surgery[1]. Consuming Lidocaine and magnesium sulfate for laparoscopic cholecystectomy has also decreased the need for opioids and painkillers during and after the surgery; also the magnesium receiving group experienced better sleep[1,2]. Also the effect of magnesium sulfate has been studied in patients undergoing women’s specialized surgeries and it was revealed that the magnesium receiving group required less chromium during hysterectomy. Also patients’ pain intensity was lower after the surgery and they required fewer painkillers and experienced a better quality sleep[1,3]. This matter was also approved in the present study. The effectiveness of magnesium sulfate which delayed the first request for painkiller has been approved previously and this matter was also confirmed for pregnant women in the present study[9,19]. Some studies revealed no significant effect of magnesium sulfate on pain reduction after surgery[15,16] but since they used general anesthesia, their results could not be compared with the results of the present study. On the other hand, in the present study, besides pain reduction, the interval before tolerating oral liquids was decreased too which is one of the benefits of magnesium sulfate especially in caesarean section; this has been approved in previous studies too. Also the serum level of magnesium was not in the toxic range in the present study. Since the stress of surgery would increase the sympathetic activities, it would inhibit bowel movements[17]. Magnesium sulfate with its lysissympathoeffect could be effective on reduction of ileus after surgery[14] and the results of the present also approved this matter.
By and large, our study is comparable with other studies[20,21] which determine perioperative intravenous magnesium sulfate (bolus or infusion) reduce analgesic consumption and pain score in the first 24 hours postoperative without any serious side effects.
In a recent study[22] the analgesic effect of magnesium sulfate was compared with ketorolac in laparascopic surgery. The results emphasized that intravenous magnesium sulfate reduced morphine consumption and pain intensity similar to ketorolac. As the safety of magnesium sulfate usage is a priority of this drug in comparison of other painkillers with serious side effects, this study can recommend usage of this drug to reduce pain and also ileus time after cesarean surgery in parturient.
One of the limitations of the present study was not measuring the magnesium level of cerebrospinal fluid (CSF) and it is suggested to consider it in further studies. Another limitation in this study was the problems the patients had with the loading dose infusion, which means they had nausea and vomiting and complained for flushing. So we had to slow the infusion rate more than 10 minutes.
Conclusion
The present study resulted that intravenous infusion of magnesium sulfate ((loading dose 40 mg/kg and continuing with 15 mg/kg/h) during surgery, with the least side effects, could be used in caesarean sections with spinal anesthesia and would definitely decrease pain intensity and duration of ileus after surgery and would also increase the analgesic period and the time of requesting painkillers by the mothers. Also prescription of this drug had no adverse effect on Apgar score of the neonates.
Acknowledge:
The authors wish to sincerely thank the support of all the colleagues in Baheshti Hospital Medical Center affiliated to Isfahan University of Medical Sciences in Isfahan, Iran. Furthermore, our special thanks go to the patients, who wholeheartedly and actively assisted us to carry out this research. No conflict of interest existed. This prospective randomized observational study was approved by the Ethics Committee of our university, (Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran) and all patients gave written, informed consent.
Conflict of interest: Authors have no conflict of interests.
Financial Disclosure: Anesthesiology and Critical Care Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1. Sekhavat, L., Behdad, S. Preoperative analgesia with local lidocaine for cesarean delivery pain relief. (2011) J Matern Fetal Neonatal Med 24(7): 891-893.
Pubmed || Crossref - 2. Navali, N., Fouladi, R., Nikpour, M. A comparison of post-incisional subcutaneous, intramuscular, and subcutaneous plus intramuscular infiltrations of lidocaine in postcaesarean section pain control. (2013) South African Journal of Obstetrics and Gynaecology 19(1).
Crossref - 3. Moharari, R.S., Motalebi, M., Najafi, A., et al. Magnesium can decrease postoperative physiological ileus and postoperative pain in major non laparoscopic gastrointestinal surgeries: a randomized controlled trial. (2013) Anesth Pain Med 4(1).
Pubmed - 4. Ryu, J.H., Kang, M.H., Park, K.S., et al. Effects of magnesium sulphate on intraoperative anaesthetic requirements and postoperative analgesia in gynaecology patients receiving total intravenous anaesthesia. (2008) Br J Anaesth 100(3): 397-403.
Pubmed || Crossref - 5. Saadawy, I., Kaki, A., Abd-El-Latif, A., et al. Lidocaine vs. magnesium: effect on analgesia after a laparoscopic cholecystectomy. (2010) Acta Anaesthesiol Scand 54(5): 549-556.
Pubmed || Crossref - 6. Petrenko, A.B., Yamakura, T., Baba, H., et al. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. (2003) Anesth Analg 97(4): 1108-1116.
Pubmed || Crossref - 7. McCartney, C.J., Sinha, A., Katz, J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia. (2004) Anesth Analg 98(5): 1385-1400.
Pubmed || Crossref - 8. Koinig, H., Wallner, T., Marhofer, P., et al. Magnesium sulfate reduces intra-and postoperative analgesic requirements. (1998) Anesth Analg 87(1): 206-210.
Pubmed - 9. Hwang, J.Y., Na, H.S., Jeon, Y.T., et al. IV infusion of magnesium sulphate during spinal anaesthesia improves postoperative analgesia. (2010) Br J Anaesth 104(1): 89-93.
Pubmed || Crossref - 10. Albrecht, E., Kirkham, K., Liu, S.S., et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta‐analysis. (2013) Anaesthesia 68(1): 79-90.
Pubmed || Crossref - 11. Shariat, M.R., Motalebi, M., Najafi, A., et al. Magnesium Can Decrease Postoperative Physiological Ileus and Postoperative Pain in Major non Laparoscopic Gastrointestinal Surgeries: A Randomized Controlled Trial. (2013) Anesth Pain Med 4(1): e12750.
Pubmed - 12. Lee, D., Kwon, I. Magnesium sulphate has beneficial effects as an adjuvant during general anaesthesia for Caesarean section. (2009) Br J Anaesth 103(6): 861-866.
Pubmed || Crossref - 13. Ozcan, P.E., Tugrul, S., Senturk, N.M., et al. Role of magnesium sulfate in postoperative pain management for patients undergoing thoracotomy. (2007) J Cardiothorac Vasc Anesth 21(6): 827-831.
Pubmed || Crossref - 14. Fawcett, W., Haxby, E., Male, D. Magnesium: physiology and pharmacology. (1999) Br J Anaesth 83(2): 302-320.
Pubmed || Crossref - 15. Paech, M.J., Magann, E.F., Doherty, D.A., et al. Does magnesium sulfate reduce the short-and long-term requirements for pain relief after caesarean delivery? A double-blind placebo-controlled trial. (2006) Am J Obstet Gynecol 194(6): 1596-1602.
Pubmed || Crossref - 16. Ko, S.H., Lim, H.R., Kim, D.C., et al. Magnesium sulfate does not reduce postoperative analgesic requirements. (2001) Anesthesiology 95(3): 640-646.
Pubmed || Crossref - 17. Fotiadis, R., Badvie, S., Weston, M., et al. Epidural analgesia in gastrointestinal surgery. (2004) Br J Surg 91(7): 828-841.
Pubmed || Crossref - 18. Schulz-Stübner, S., Wettmann, G., Reyle-Hahn, S., et al. Magnesium as part of balanced general anaesthesia with propofol, remifentanil and mivacurium: a double-blind, randomized prospective study in 50 patients. (2001) Eur J Anaesthesiol 18(11): 723-729.
Pubmed || Crossref - 19. Apan, A., Buyukkocak, U., Ozcan, S., et al. Postoperative magnesium sulphate infusion reduces analgesic requirements in spinal anaesthesia. (2004) Eur J Anaesthesiol 21(10): 766-769.
Pubmed || Crossref - 20. Albrecht, E., Kirkham, K.R., Liu, S.S., et al. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: a meta-analysis. (2013) Anaesthesia 68(1): 79-90.
Pubmed || Crossref - 21. Shin, H.J., Kim, E.Y., Na, H.S., et al. Magnesium sulphate attenuates acute postoperative pain and increased pain intensity after surgical injury in staged bilateral total knee arthroplasty: a randomized, double-blinded, placebo-controlled trial. (2016) Br J Anaesth 117(4): 497-503.
Pubmed || Crossref - 22. Sousa, A.M., Rosado, G.M., Neto, J.D.S., et al. Magnesium sulfate improves postoperative analgesia in laparoscopic gynecologic surgeries: a double-blind randomized controlled trial. (2016) J Clin Anesth 34: 379-384.
Pubmed || Crossref