In Vivo Imaging of Lung Tumor Growth Using In-Line X-Ray Phase Contrast Technique
Rongbiao Tang1*, Fuhua Yan1, Guo-Yuan Yang2, Ke-Min Chen1*
Affiliation
- 1Department of Radiology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
- 2Neuroscience and Neuroengineering Center, Med-X Research Institute, Shanghai Jiao Tong University, Shanghai 200030, China
Corresponding Author
Rongbiao Tang, Department of Radiology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China, E-mail: tangme8688258@sina.com &
Department of Radiology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China, E-mail: keminchenrj@163.com
Citation
Tang, R., et al. In vivo imaging of lung tumor growth using in-line x-ray phase contrast technique. (2016) J Imaging Sci 3(1): 174-176.
Copy rights
© 2016 Tang, R. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Lung cancer; Synchrotron radiation (SR); Phase contrast imaging (PCI); Absorption contrast imaging (ACI)
Abstract
The primary purpose of present study was to investigate the application value of Synchrotron Radiation Phase Contrast Imaging (SR-PCI) for the evaluation of lung tumor growth. Comparison between Absorption Contrast Imaging (ACI) and inline PCI was observed in living tumor-bearing mice. Early lung cancer was shown at pre-injection, 12 h and 36 h post-injection in the same mouse. PCI was performed to noninvasively monitor tumor progression in a longitudinal study. It was concluded that PCI was a helpful imaging modality for detecting early lung tumor and monitoring tumor growth in mouse models.
Introduction
Lung cancer is the primary cause of cancer-related mortality across the world[1]. More than 85% of patients die within five years due to lack of early diagnosis and effective treatment[2]. Early tumor detection can encourage timely treatment to make a better prognosis. Clinically, a chest radiograph is often carried out if lung cancer is suspected. However, conventional x-ray imaging techniques can hardly identify lesions less than 1 cm in diameter. CT has a high contrast resolution to reveal more details, but it cannot detect minor pathological changes of early tumors[3]. It is clear that lung cancer is often detected relatively late in its natural course by using conventional x-ray imaging techniques[4].
Synchrotron Radiation (SR) has high brightness, high intensity and high collimation[5]. SR imaging can give high spatial resolution down to the sub-micron scale. In addition, SR imaging could afford millisecond-level temporal resolution. Therefore, SR imaging can capture clear images for rapidly moving organs, such as mouse lungs. Besides absorption, phase shift is another essential contrast mechanism between x-rays and tissues. Phase Contrast Imaging (PCI) can use phase shift to show the tissues approximately 1000 times more clearly than Absorption Contrast Imaging (ACI)[6]. The phase contrast effect deeply raise visibility of interfaces between two media with different refractive indices[7]. Due to the existence of air–tissue interface, PCI has been applied to produce greatly boundary-enhanced images in lung[6]. Therefore, it may be capable to clearly discriminate cancerous tissues from normal lung tissues[5].
In this study, ACI and PCI were performed and compared, and then PCI was used to noninvasively visualize tumor development in vivo.
Materials and Methods
Cell line
LLC (Lewis lung carcinoma) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and cells were maintained at 37° in a humidified of 5% CO2 in air.
Intrapulmonary implantation procedure
The study was conducted in accordance with the standards established by the Guidelines for the Care and Use of Laboratory Animals of Shanghai Jiao Tong University (SJTU). 30 male C57BL/6 (6 - 8 weeks old) mice were purchased from the Animal Center, Chinese Academy of Sciences (CAS), Shanghai, China. Animals had free access to standard laboratory mouse food and water ad libitum. Mice were anesthetized with an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. 5 mice were used as control group. The other 25 mice received an intrapulmonary inoculation of LLC cells, and then were randomly divided into 5 groups of 5 animals each. The first group was used to perform the comparison of ACI and PCI. The second group was used to image early lung cancer. The other three groups were used to image the tumors at days 3, 5 and 7 after cell implantation, respectively. Models were made according to previous research with some modifications[8]. Briefly, skin disinfection was performed with 70% alcohol and a small incision was made on the left chest wall. Sub-skin tissue was separated from ribs until the pleura were reached. Subsequently, after a 29-gauge needle was inserted into the left lung to an approximate depth of 3 mm, 2 × 106 cells in 0.1 ml suspension containing matrigel basement membrane matrix (Becton Dickinson, USA) were injected into the lung parenchyma rapidly through the incision. The mice recovered on a heating pad (RWD life science, Shenzhen, China). The mice were put back in the cage after they were fully awake.
SR parameters
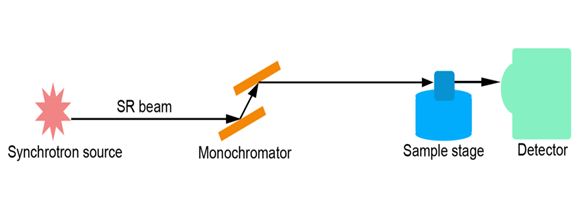
Imaging was performed at the beamline BL13W1, SSRF (Shanghai Synchrotron Radiation Facility), China. The SR beam line was produced from a storage ring of electrons with an accelerated energy of 3.5 GeV and an average beam current of 180 mA. It could supply photon energy with a range of 8 - 72.5 keV. Imaging was performed by using a thin (100 μm) CdWO4 cleaved single-crystal scintillator and a detector (Photonic Science, Britain). X-rays were monochromatized by a double- crystal monochromator with Si(111) and Si(311) crystals. The energy resolution was ΔE/E < 5 × 10-3. Samples were placed 34 m downstream of the point source from the electric storage ring. The distance between the sample and the detector could be changed from 0 to 8 m. A diagram of the setup is presented in Figure 1.
Figure 1: Scheme of the experimental set-up of SR imaging system at the beamline BL13W1. The distance from the source of light to the specimen was 34 m. The distance between the specimen and detector could be changed from 0 to 8 m.
PCI in vivo
The anaesthetized mice were suspended on a bracket that was vertical to the x-ray beam. The object-to-detector distance for ACI and PCI was 1 cm and 100 cm, respectively. Comparison of ACI and PCI was performed in 5 mice with 3 days-tumor inoculation. All SR imaging was performed at an energy level of 19 keV. Early lung cancer was observed at pre-injection, 12 h and 36 h post-injection. Further tumor development was monitored at days 3, 5 and 7 after cell implantation.
Image processing
In order to remove background noises produced by the CCD camera, original data were processed with Image Pro Plus 6.0 (Media Cybernetics Inc, USA). Images were joined together to obtain a whole view of the sample by using Adobe Photoshop CS4 (Adobe Systems Inc, USA).
Results
Comparison of ACI and PCI
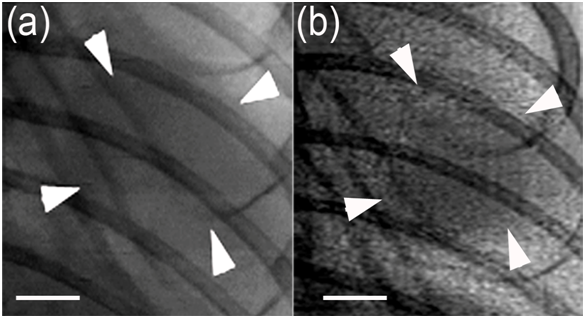
Absorption contrast image and phase contrast image of a living tumor-bearing mouse were shown in Figure 2. Lung tumor could be more distinctly visualized in phase contrast image (Figure 2b) than in the absorption image (Figure 2a). PCI was found to more sensitively detect lung tumor in vivo.
Figure 2: In vivo SR images of the same Lewis lung tumor at 19 keV with two object-to-detector distances of 1 cm (a) and 100 cm (b). Imaging was performed on day 3 after tumor inoculation. Note that the tumor could be more clearly visualized in phase contrast image (b) than in the absorption image (a). The pixel size was 13 μm × 13 μm; the exposure time was 6 ms. Arrowheads indicate the tumor. Scale bars: 1 mm.
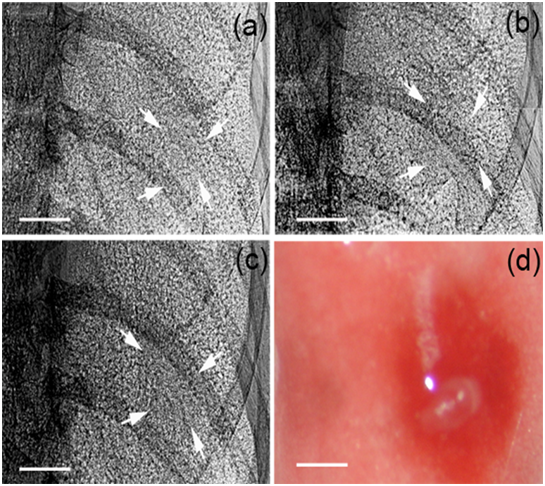
Figure 3: Early lung tumor detection by PCI. PCI was performed at pre-injection (a), 12 h (b) and 36 h post-injection (c) in the same mouse. A longitudinal increase in density at cell injection site could be observed over the first 36 h time period. The lung tumor was seen in dissected lung (d). The pixel size was 9 μm × 9 μm; the exposure time was 10 ms. Arrowheads indicated LLC cell injection sites. Scale bars: 1 mm.
PCI of tumor growth in vivo
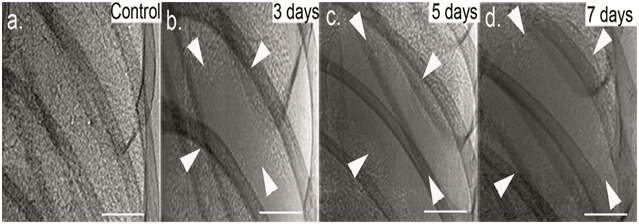
Early tumor growth could be noninvasively monitored by PCI in Figure 3. Lung cancer had a density higher than surrounding normal tissues, and a time-dependent increase in density could be observed in the first 36 h time period after cell inoculation. So PCI helped distinguish cancerous tissues from normal lung tissues in early-stage tumor. In addition, further tumor progression could be longitudinally observed by PCI (Figure 4).
Figure 4: Tumor progression analysis by PCI. The living C57BL/6 mice were monitored for lung tumor growth by PCI at days 3 (b), 5 (c) and 7 (d) after inoculation of LLC cells. PCI demonstrated a longitudinal increase in tumor size. The pixel size was 9 μm × 9 μm; the exposure time was 10 ms. Arrowheads indicate the tumor dimensions. Scale bars: 1 mm.
Discussion
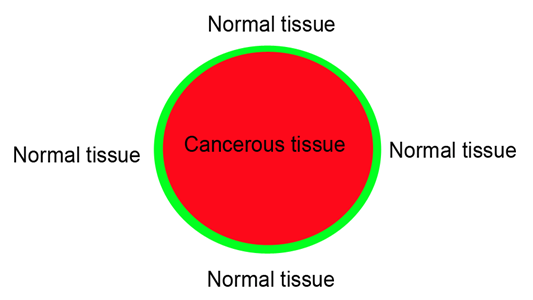
ACI makes use of beam attenuation as the imaging signal; however, the x-ray absorption contrast is often weak in soft tissues. Though lung cancer tissues had a relatively higher density than normal lung tissues, the tiny difference between the absorption coefficients of them made only little contrast on the absorption image. According to the in-line PCI principles, phase contrast can be acquired by increasing the object-to-detector distance[9]. After increasing the object-to-detector distance, the edge of the sample can be clearly displayed. Air-filled normal lung tissue will be damaged if a tumor happens. Hence, air–soft tissue interface (Figure 5) between the normal lung tissue and cancerous tissue will create highly boundary-enhanced effect, which helps to make the tumor boundary highly defined. Therefore, the phase contrast effect can make the cancerous tissue clearly stand out from its surrounding normal tissue.
Figure 5: A cartoon depiction of air-soft tissue interface (green) between the normal lung tissue (white) and cancerous tissue (red). Airfilled normal lung tissue is damaged if a tumor happens, then the air-soft tissue interface will create highly boundary-enhanced effect with PCI
Cancer cells divided uncontrollably and then formed a mass of tissue. Over time, the tumor mass became more and more dense. In the early stage of the cancer, density change was always too tiny to be shown by ACI; however, the phase shift change was large enough to be detected by PCI. The rapid image acquisition system contributed to the success of clear revelation of different-stage lung tumors in vivo. The exposure time for each capture was only 6 ms when using the 13-μm detector (Figure 2). However, the spatial resolution was inferior to the 9-μm detector. The exposure time for each capture was 10 ms when using the 9-μm detector (Figure 3,4). This short exporsure time could ensure the ability to well control potential artifacts from moving and breathing of the mice. It took about 5 minutes to complete the whole image acquisition for each mouse. Using PCI, a time-dependent increase in density at cell injection site could be undoubtedly observed (Figure 3). Therefore, it was feasible to utilize PCI to detect early-stage lung cancer. Tumor growth in lung was shown by PCI in a one-week follow-up study (Figure 4). This application can be used to judge whether tumor models succeed, and preliminarily evaluate the efficacy of tumor therapy by observing tumor dimensions. However, in order to determine the tumor volume more precisely, 3D visualization of the tumor is required. Because the rotating sample stage is too small to allow the location of the living mouse, only 2D projection radiography, rather than 3D CT imaging, could be performed in our experimental hutch. Therefore, the current two-dimensional PCI may be considered as a complementary tool for tumor growth.
Acknowledge:
We thank the National Science Foundation of China (grants 81301347, 81271740 and 81471808), the National Basic Research Program of China (973 Program 2010CB834305), and SJTU Med-Science Cross Research Foundation (YG2013MS30).
Conflict of interest:
authors declare that they have no conflicts of interest.
References
- 1. Jemal, A., Murray, T., Samuels, A., et al. Cancer statistics, 2003. (2003) CA Cancer J Clin 53(1): 5-26.
- 2. Hirsch, F.R., Franklin, W.A., Gazdar, A.F., et al. Early Detection of Lung Cancer: Clinical perspectives of recent advances in biology and radiology. (2001) Clin Cancer Res 7(1): 5-22.
- 3. Lynch, D., Newell, J., Lee, J. Imaging of diffuse lung disease: Pmph Bc Decker, 2000.
- 4. Kitchen, M.J., Lewis RA, Yagi N, et al. Phase contrast X-ray imaging of mice and rabbit lungs: a comparative study. (2005) Br J Radiol 78(935): 1018-1027.
- 5. Liu, P., Sun, J.Q., Guan, Y.J., et al. Morphological study of early-stage lung cancer using synchrotron radiation. (2008) J Synchrotron Rad 15: 36-42.
- 6. Lewis, R.A. Medical phase contrast x-ray imaging: current status and future prospects. (2004) Phys Med Biol 49(16): 3573-3583.
- 7. Davis, T.J., Gao, D., Gureyev, T.E., et al. Phase-contrast imaging of weakly absorbing materials using hard X-rays. (1995) Nature 373(6515): 595-598.
- 8. Doki, Y., Murakami, K., Yamaura, T., et al. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. (1999) Br J Cancer 79(7-8): 1121-1126.
- 9. McDonald, S.A., Marone, F., Hintermüller, C., et al. Advanced phase-contrast imaging using a grating interferometer. (2009) J Synchrotron Radiat 16(Pt 4): 562-572.