Screening of Potential Bioactive Compounds from Padina gymnospora found in the Coast of St. Martin Island of Bangladesh
Md. Farzanoor Rahman,Md. Abdul alim,Tamim Ahsan,Taufiqul Islam,Md. Morshedul Alam
Affiliation
Department of Genetic Engineering and Biotechnology, Faculty of Earth and Ocean Science, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka-1216, Bangladesh.
Corresponding Author
Mohammad Nazir Hossain, Dept. of Genetic Engineering & Biotechnology, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka-1216; E-mail: nazir.geb@bsmrmu.edu.bd
Citation
Nazir Hossain, M.D., et al. Screening of Potential Bioactive Compounds from Padina Gymnospora Found In the Coast Of St. Martin Island Of Bangladesh. (2021) J Marine Biol Aquacult 6(1): 1-7.
Copy rights
© 2021 Nazir Hossain, M.D. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Keywords
Bioactive compounds; Padina gymnospora; Antimicrobial activity; Antioxidant; Cytotoxic; St. Martin Island
Abstract
Seaweed is the most popular marine resource which plays an important role biologically in different research area in the world. In spite of this significance of seaweed very few algae based studies have been conducted in Bangladesh. For the first time this study aimed to find out the potential bioactivity of Padina gymnospora collected from the St. Martin Island of Bangladesh. The present study investigated the potential bioactivities of Padina gymnospora. Initially Study subject was extracted with five different solvents. Crude extracts were screened for crucial phytochemicals. DPPH radical scavenging activity was implemented for antioxidant test. The antibacterial activities were examined by agar well diffusion method. Cytotoxic test were conducted following Brine Shrimp Lethality Assay. The result showed that Methanol and ethanol crude extracts exhibited the presence of the highest number of bioactive compounds. The highest % inhibition (48.28 ± .47) along with a significant IC50 value of 369.99 µg/ml (p<0.05) was found in ethanol crude. Isopropanol followed by methanol crude extract has shown strong activity against B. cereus, S. aureus. Chloroform crude extract was highly effective against Salmonella species while ethanol crude extract showed the highest activity against S. aureus, Salmonella species and S. hominis. It is also recorded that the isopropanol and chloroform crude extracts showed highly cytotoxicity (IC50<100μg /ml) (p<0.05). The present findings suggested that Padina gymnospora has substantial bioactivity due to the presence of phenolic compounds, flavonoids, steroids and other secondary metabolites.
Introduction
Seaweed is one of the major marine sources considering its various potential aspects in different areas of research in the world[1]. Researchers from different countries are looking forward to fight back with various complications like aging problems, microbial attack, cancer as well as malnutrition using natural products isolated from different marine sources[2]. Now-a-days seaweeds found in Bangladesh, 193 species where approximately 140 species found in different region of St. Martin Island, appear to be a substantial marine living existence[3].
Different bioactive properties like antioxidant[4,5] antibacterial[6,7], hemolytic activity[8], cytotoxic[9] found in seaweed have received major attention from different parts of the diversely blue world[4-11]. Especially their least side effect along with negligible health risk leads themselves to the pinnacle of biological research and drugs industry[12].
Nevertheless, the bioactivity along with potential bioactive compound based study on Padina gymnospora, a brown seaweed, found in the coastal area at St. Martin Island of Bangladesh, has not been studied yet[3]. Therefore, our present study was undertaken to investigate its pharmacological properties by focusing on the antioxidant potential, antibacterial activity, cytotoxic effect of methanol, ethanol, aqueous, chloroform and isopropanol crude extract of P. gymnospora along with the screening of its major bioactive compounds.
Materials and Methods
Sample Collection and Processing
Study samples were collected at the end of November, 2019 from the Chera Island (Chera Dwip), an extension of St. Martin Island of Bangladesh. Sample was cleaned properly later drowned in 50% ethanol for the preservation. The collected sample was identified as Padina gymnospora based on its morphology[13]. After four weeks sample was washed thoroughly with sterile distilled water in the laboratory subsequently air-dried in the dark condition and oven dried at 37ºC, cut into small pieces later ground with mortar and pestleuntil reach fine powder shape.
Extract Preparation
3.0g of powdered sample was extracted in five different solvents (30 ml of 70% methanol, 50% ethanol, distilled water, chloroform, isopropanol) individually under shacking condition (25ºC and 150 rpm) inside of a shaking incubator (Model : JSSI-070C, JSR, Korea) for 2 hours. The solution was filtered through Whatman No. 1 sterile filter paper (Qualitative, 102). The filtrates were dried using oven at 40ºC and the dried precipitates were dissolved in the above five solvents. Concentrations for the extracts were 7.10 mg/mL, 7.05 mg/mL, 6.27 mg/mL, 6.82 mg/mL 5.96 mg/mL for methanol, ethanol, distilled water, chloroform, isopropanol crude respectively. Later crude extracts were stored in airtight bottles at -20ºC in a refrigerator before testing bioactivity.
Phytochemical Screening
Standard qualitative phytochemical procedures[14-17] were followed to detect the presence of potential bioactive compounds in the sample. Phenolic compounds, tannins, alkaloids, steroids, glycosides, flavonoids, and saponins were subjected to identify in the collected sample. Test for Phenolic compounds using lead acetate, for alkaloids using Mayer’s reagent, for tannins using ferric chloride, for flavonoids using alkaline reagent, for steroids as well as glycosides using Salkowski’s test. Saponins test done by distilled water with vigorous shaking[18].
Antioxidant Activity–DPPH Assay
The free radical inhibition activity of the four different extracts (methanol, ethanol, aqueous and chloroform) was determined using DPPH(2,2-diphenyl-1-picrylhydrazyl) following the well-established method with slight modification[10,11,19,20]. 4mg DPPH (Cat. No.: sc-202591, Santa Cruz Biotechnology, USA) was mixed with 100 mL of 95% methanol.3 mL of individual mixture (Crude extract: DPPH = 1:1) was prepared where four different crude extracts were subjected to dilution separately for making the concentration of 12.5µg/mL,25 µg/mL, 50µg/mL, 100µg/mL, 200µg/mL and 400 µg/mL for each of the samples. Later the mixtures were incubated for about 1 hour in a dark condition and the decrease in absorbance was monitored at 517 nm in UV-VIS spectrophotometer (Model: UV-1900, Shimadzu). Ascorbic acid is used as a standard. The percentage of scavenging (%SCV) was calculated as:
% SCV = 1 -(A 1 – A 0)/ (A 2– A 0) x 100
Where,
A1= Absorbance of sample
A2= Absorbance of control
A0= Absorbance of blank
Cytotoxic Activity-Brine Shrimp Lethality Assay (BSLA)
Cytotoxic activity was investigated following with slight modification of previously published method[10,11,21-23]. The 150mg of Brine shrimp (Artemiasalina) eggs were hatched in 1L sea water (pH=8.31) for 24 hours under continued aeration in a cylindrical container illuminated by a 60 watts bulb to produce nauplii. Six different concentrations of 45, 90, 135, 180, 225 and 270µg/ml of sample extracts were prepared in each of the solvents of methanol, ethanol, distilled water, chloroform and isopropanol. Later 3 ml were transferred in Eppendorf and left open for 48 hours to evaporate the organic solvent before adding the nauplii. After 24 hours, the phototropic nauplii were collected. Ten brine shrimp nauplii were transferred in each of six test tubes. Four mL of seawater was added in each test tube of five different extract of the same samples before adding the nauplii. After 24 hours the observations were noted and survivors were counted and percent death at each dose level was calculated as:
Mortality (%) = (Number of dead nauplii)/X 100
(Number of dead nauplii + Number of alive nauplii)
Potassium dichromate (K2Cr2O7) was used as positive control and the above mentioned five different solvents were used as negative control. Concentration and the cytotoxic test for the positive control were similar as mentioned above and all the cytotoxic tests were performed in triplicate.
Antibacterial Activity Assay
Antimicrobial assay was performed by agar well diffusion method in Luria Bertani (LB) media and the minimum inhibitory concentration of those extract was determined by dilution method. All the strains of bacteria was cultured in LB broth and incubated at 37°C for 18 hours in incubator (Model – JSGI - 050T, JSR, Korea). After incubation each stain were diluted with sterile distilled water. Prepared inoculums were incubated for 30 minutes at 37°C prior to use. Crude extracts (150μl) were loaded into the respective wells. Solvents (Methanol, ethanol, distilled water, chloroform and isopropanol) were tested for their activity as negative control at the same time in the separate petridishes. The Ampicillin 20µg/ml, Tetracycline 20µg/ml, Kanamycin 20µg/ml, Ciprofloxacin 20µg/ml was used as a positive control. The Petridishes were then left for an hour with the lid closed so that extracts diffused to the media. The plates were incubated overnight at 37°C. After proper incubation (18 hours) the plates were observed for the zone of inhibition (ZOI) around well which is suggested by clean zone without growth. The ZOI were measured with the help of the ruler and mean was recorded for the calculation.
Determination of Minimum Inhibitory Concentration(MIC)
A stock solution of 0.5 mg/ml was prepared for five different crude extract (methanol, ethanol, aqueous, chloroform, isopropanol) of the study samples. This was serially diluted to obtain various ranges of concentrations between 0.5 mg/ml to 0.020 mg/ml.
Statistical Analysis
All data found in this study were expressed as mean values±SD of triplicate. Using one-way ANOVA the mean values were analyzed. The means of parameters were determined significantly (p<0.05). Statistical Packages for Social Sciences ‘IBM SPSS statistics-20’ Software, Microsoft Excel programme 2007 were used for the data analysis. LC50 was determined using Probit analysis.
Results
Bioactive Compounds Screening
Phenolic compounds, flavonoids, steroid and tannins found maximum in ethanol crude extract of P. gymnospora followed by Chloroform and methanol crude extract while aqueous crude had all the tasted phytochemicals in a trace amount except the absence of alkaloid and saponins. The qualitative analysis of phytochemicals of four different crude extracts P. gymnospora is given in Table 1.
Table 1: Qualitative phytochemical analysis of crude extracts of P. gymnospora.
|
Attributes |
Methanol |
Ethanol |
Aqueous |
Chloroform |
|
Crude Extract |
Crude Extract |
Crude Extract |
Crude Extract |
|
|
Tannins |
+ |
++ |
+ |
++ |
|
Phenolic compounds |
++ |
+++ |
+ |
++ |
|
Alkaloids |
+ |
++ |
- |
- |
|
Flavonoids |
++ |
+++ |
+ |
++ |
|
Saponins |
+ |
- |
- |
+ |
|
Glycoside |
+ |
+ |
+ |
- |
|
Steroid |
+ |
+++ |
+ |
++ |
[N.B. n=3, (-): not detectable, (+): low quantities, (++): moderate quantities, (+++): high quantities]
Antioxidant Activity–DPPH Assay
Free radical scavenging is important to maintain the redox homeostasis[24]. In this study, the antioxidant activity of the samplewas determined using DPPH method. The reaction mixture color gradually changed into yellow from the initial purple color. The scavenging activity of methanol, ethanol, aqueous, chloroform extracts were compared with the standard ascorbic acid. Ethanol crude extract showed the highest antioxidant activity followed by methanol, chloroform and aqueous crude extract of P. gymnospora. The existent antioxidant of four different crude extracts level in the selected seaweed is mentioned in Table 2 and illustrated in figure 1(a, b). The IC50 value was also calculated where it also followed the similar tendency of efficacy with the highest IC50 value of ethanol extract.
Table 2: Antioxidant activities of P. gymnospora extracts.
|
Conc. (µg/mL) |
% of Inhibition |
||||
|
Positive control |
Different crude extracts of P. gymnospora |
||||
|
Ascorbic Acid |
50% Ethanol |
Chloroform |
70% Methanol |
Aqueous |
|
|
12.5 |
10.01 ± 0.36 |
5.28 ± 0.37* |
3.99 ± 0.29 |
3.19 ± 0.29 |
3.01 ± 0.14 |
|
25 |
17.84 ± 0.20 |
9.99 ± 0.19* |
5.56 ± 0.16 |
5.01 ± 0.24 |
4.45 ± 0.42 |
|
50 |
25.46 ± 0.25 |
18.74 ± 0.14* |
8.45 ± 0.42 |
7.99 ± 0.19 |
6.82 ± 0.54 |
|
100 |
50.99 ± 0.66 |
29.99 ± 0.24* |
14.74 ± 0.26 |
12.01 ± 0.34 |
9.99 ± 0.34 |
|
200 |
77.01 ± 0.38 |
38.99 ± 0.53* |
19.45 ± 0.35 |
16.74 ± 0.53 |
12.28 ± 0.62 |
|
400 |
77.70 ± 0.49 |
48.28 ± 0.47* |
23.19 ± 0.24 |
19.56 ± 0.26 |
14.74 ± 0.43 |
N. B. Comparisons were made between ethanol extract groups with other three extract groups. Each value is presented as mean ± SD, (n=3), (*p < 0.05).

Figure 1: Antioxidant activity of P. gymnospora through DPPH assay. (a) Percentage inhibition of positive control (aa = Ascorbic Acid), (b) Percentage inhibition of four different crude extracts of P. gymnospora (b = EtOH, C = Chloroform, D = Methanol, E = Aqueous).
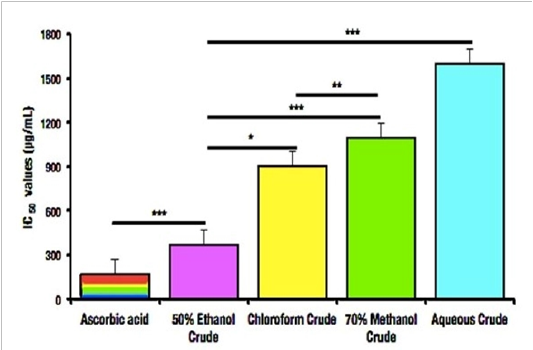
Figure 2: IC50 values of DPPH Assay determined from P. gymnospora.
Cytotoxic activity-Brine Shrimp Lethality Assay
According to the cytotoxicity test in this experiment, the mortality (%) of brine shrimp nauplii against the different crude extracts of the study sample at various concentrations were different along with those of the positive control, Potassium Dichromate (K2Cr2O7), (Table 3) indicates the future significance of the use of P. gymnospora in the treatment of cancer.
Table 3: Mortility (%) of P. gymnospora, Potassium Dichromate (positive control) and the negative control.
|
Concentration (µg/mL) |
Potassium Dichromate |
Methanol Crude Extract |
Ethanol Crude Extract |
Aqueous Crude Extract |
Chloroform Crude Extract |
Isopropanol Crude Extract |
|
45 |
10% |
10% |
10% |
0% |
30% |
20% |
|
90 |
20% |
20% |
20% |
10% |
50% |
50% |
|
135 |
30% |
30% |
30% |
10% |
60% |
70% |
|
180 |
40% |
40% |
40% |
20% |
60% |
80% |
|
225 |
50% |
60% |
50% |
30% |
80% |
80% |
|
270 |
60% |
70% |
50% |
30% |
90% |
90% |
|
Negative control showed no mortility |
||||||
The 50 percent (%) Lethal Concentration (LC) of the study sample varies with the different crude extracts of the study sample depicted in figure 3.
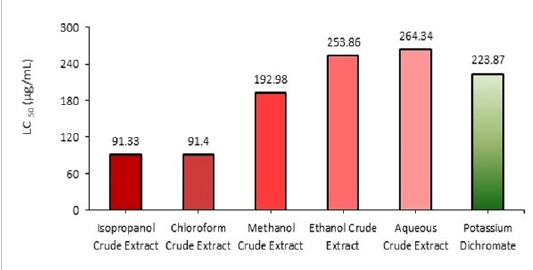
Figure 3: LC50 Value of P. gymnospora.
According to the prominent Clarkson index of the cytotoxicity[25], the toxicity status of different crude extracts of P. gymnospora was determined.
Table 4: Toxicity Profile of Padinagymnospora.
|
Extracts |
Status of toxicity |
|
Methanol crude |
Medium toxic |
|
Ethanol crude |
Medium toxic |
|
Aqueous crude |
Medium toxic |
|
Chloroform crude |
Highly toxic |
|
Isopropanol crude |
Highly toxic |
Antibacterial activity assay
The antimicrobial activities of the tested crude extracts of P. gymnospora were evaluated by agar well diffusion method and the results were expressed as the diameter of inhibition zone (mm). Most of the extracts of P. gymnospora showed antimicrobial potency against the selected bacteria.
Determination of Minimum Inhibitory Concentration (MIC):
The smallest amount of compounds required to kill or inhibit the growth of microorganism in vitro is determined by the dilution method and considered as minimum inhibitory concentration (MIC). The MIC of five different crude extracts of the study sample against the tested organism is mentioned in the table 6.
Table 6: Overview of MIC along with Zone of inhibition (ZOI) of P. gymnospora.
|
Tested organism |
Attribute |
MIC according to ZOI |
||||
|
70% MeOH |
50% EtOH |
Aqueous |
Chloroform |
Isopropanol |
||
|
Bacillus cereus |
MIC |
50 µg/ml |
Nil |
70 µg/ml |
40 µg/ml |
30 µg/ml |
|
Staphylococcus aureus |
MIC |
50 µg/ml |
40 µg/ml |
Nil |
50 µg/ml |
30 µg/ml |
|
Klebsiella pneumonia |
MIC |
60 µg/ml |
50 µg/ml |
Nil |
40 µg/ml |
40 µg/ml |
|
Salmonella species |
MIC |
60 µg/ml |
40 µg/ml |
Nil |
30 µg/ml |
40 µg/ml |
|
Salmonella paratyphi |
MIC |
Nil |
60 µg/ml |
Nil |
Nil |
50 µg/ml |
|
Staphylococcus hominis |
MIC |
Nil |
40 µg/ml |
Nil |
Nil |
60 µg/ml |
N.B. MIC- Minimum Inhibitory Concentration (µg/ml), ZOI-Zone of Inhibition (mm).
Discussion
In the current study, we attempted to find out and characterize the major bioactive compounds found in Padina gymnospora using various different solvents. In addition, we also appraised its antioxidant, cytotoxic and antibacterial activity to establish the pharmacological significance. We confirmed the presence of tannins, phenolics, flavonoids, and steroids in all of the types of extracts from P.gymnospora, notably methanol and ethanol crude extracts contained higher amount of these bioactive compounds, which is similar with the previous finding though the species was different[26,27]. Saponins, alkaloids and glycosides were also the additive findings of the methanol and ethanol extracts (Table 1). The presence of these bioactive compounds suggests the pharmacological properties of the P.gymnospora, which leads the subsequent antioxidant, cytotoxic and antimicrobial studies.
In the living system, free radicals are generating frequently as a response to metabolism[28,29] and these free radicals are also scavenged by antioxidants that lead redox homeostasis in the body[24]. Aberration of redox homeostasis compels various health hazards like cancer, diabetes, arthritis, neurological disorders etc[30]. Seaweeds are the well-heeled sources of antioxidant[31], and in our study, we also found significant amount of antioxidant response through DPPH assay. The 50% ethanol crude extract of P. gymnospora is substantially effective (p< 0.05) at the IC50 level than other crude extracts in the present study. The 50% ethanol crude extract of this species had significantly more activity compared with all the concentrations used in the experiment (p<0.05) than the chloroform crude extract (p<0.05), 70% methanol crude extract (p<0.05), aqueous crude extract of P. gymnospora (p<0.05) (Fig. 1b, Fig.2; Table 2), which was similar with the same genus but different species[32]. It is well known that flavonoids, alkaloids and phenolic compounds, which were also common in our study, possess antioxidant activity due their capability to interact with protein phosphorylation, iron chelating, scavenging hydrogen peroxide by donating electron and thereby neutralizing it to water, absorbing and neutralizing superoxide anion (O2–•), hydroxyl radical or peroxy radicals, quenching singlet and triplet oxygen or decomposing peroxides as well as terminating the radical chain reaction involved in lipid peroxidation by converting free radicals and reactive oxygen species to more stable products[33-38]. Thus, the antioxidant activity of P. gymnospora extracts might be accredited to these modes of action due to their flavonoid, alkaloid, and phenolic contents.
Cytotoxic property of a compound is an important measure for the treatment of tumor due to its cell cycle inhibitory role. In this line, screening of some marine seaweeds with cytotoxic properties is additive in the field of chemotherapy. Generally, cytotoxic compounds present in seaweeds provide a protective defense to them from many herbivores[39]. In our study, the cytotoxic effect of the bioactive compounds present in different solvents extracts of Padina gymnospora was studied using brine shrimp lethality assay (BSLA). From the examined crude extracts of this study subject, the isopropanol and chloroform crude extracts showed the highest toxic activity in brine shrimps. The LC50 results obtained for isopropanol crude extract was 91.33µg/mL and for chloroform crude extracts 91.48µg/mL indicated higher toxicity whereas 70% methanol, 50% ethanol and aqueous crude extracts of the same sample showed the LC50 values 192.98µg/mL, 253.86 µg/mL, 264.34 µg/mL, respectively (Fig. 3; Table 3 & 4). It is well known that the phytochemical phenolic compounds and steroids are the tycoon concerning the cytotoxicity, which is also similar in line with our phytochemical analysis that suggests that the presence of these bioactive compounds would be the underlying factors for the cytotoxic properties of P. gymnospora.
The antimicrobial activity of the five different extracts of Padina gymnospora were determined by measuring the zone of inhibition in the Kirby Bauer well diffusion assay. Depending upon their solubility and polarity, different solvents shows the different antibacterial activity. The antibacterial activity of the 50% ethanol and isopropanol crude extracts of study sample showed a prospective activity against most of the pathogens. Isopropanol had higher antibacterial activity than that of extracts obtained with other organic solvents such as ethanol, methanol, chloroform, and distilled water (Table 5). The presence of phenolic contents and alkaloids may be responsible for the antibacterial activity and for more precise mechanism concerning to alkaloids, for example, sanguinarine, perturbs bacterial FtsZ-Z-ring formation and inhibits hacterial cytokinesis[40,41]. 50% Ethanol extract of P. gymnospora are active against most of the gram positive and negative bacteria. In this present study, we concluded that Isopropanol extract followed by ethanol extract showed an adequate amount of phytochemical compounds hence it is taken for antibacterial activity testing.
Table 5: Antibacterial activity of various crude extracts of P. gymnospora.
|
Test Organisms |
Diameter of the Zone of Inhibition (mm) |
||||||||
|
|
Extracts of Padinagymnospora |
Positive control |
|||||||
|
|
EtOH |
MeOH |
Aque. |
Chlor. |
Isop. |
Amp. |
Tet. |
Kan. |
Cipro. |
|
Bacillus cereus |
- |
12.00 ± 0.41***a2 |
10.50 ± 0.41*a3 |
15.00 ± 0.82**a1 |
27.50 ± 0.41a |
Nil |
8.33 ± 0.58***a4 |
16.50 ± 0.50*a5 |
19.50 ± 0.50*a6 |
|
Staphylococcus aureus |
18.17 ± 0.85***b1 |
11.17 ± 0.85*b2 |
- |
12.50 ± 0.41**b3 |
28.00 ± 0.82b |
3.67 ± 0.29 |
21.17 ± 0.76*b4 |
16.33 ± 0.29*b5 |
22.17 ± 0.76**b6 |
|
Klebsiella pneumonia |
13.00 ± 0.82**c2 |
10.45 ± 0.62 |
- |
18.50 ± 0.41c |
13.50 ± 0.41***c1 |
Nil |
7.50 ± 0.50 |
11.67 ± 0.29***c3 |
11.00 ± 0.58 |
|
Salmonella species |
14.50 ± 0.41***d1 |
10.50 ± 0.41**d2 |
- |
24.00 ± 0.82d |
10.50 ± 0.41*d3 |
Nil |
1.83 ± 0.29*d4 |
8.00 ± 0.50*d5 |
4.67 ± 0.58 |
|
Salmonella paratyphi |
09.50 ± 0.41e |
- |
- |
- |
09.50 ± 0.82e |
15.33 ± 0.76*e1 |
19.67 ± 0.58**e2 |
17.83 ± 0.29**e3 |
14.67 ± 0.58*e4 |
|
Staphylococcus hominis |
14.00 ± 0.82f |
- |
- |
- |
10.50 ± 0.41*f1 |
3.50 ± 0.5 |
20.83 ± 0.76*f2 |
19.50 ± 0.87 |
22.17 ± 0.29*f3 |
|
Zone of inhibition was not found in negative control |
|
|
|||||||
N.B. Each value is presented as mean ± SD, n=3. *p < 0.05, **p<0.01, ***p<0.001 were considered as statistically significant. Comparisons were made among a, b, c, d, e and f and their consecutive orders. EtOH – ethanol, MeOH – methanol, Aque – aqueous, Chlor – chloroform, Isop – isopropanol, Amp – ampicillin, Tet – tetracycline, Kan – kanamycin, Cipro – ciprofloxacin.
The minimum inhibitory concentration (MIC) was observed in methanol, ethanol, aqueous, chloroform and isopropanol crude extract of the study sample against the six different test organisms. The lowest MIC (30 µg/ml) was found both in isopropanol crude extract against Bacillus cereus and staphylococcus aureus bacteria and in the chloroform crude extract against Salmonella species. The highest MIC (70 µg/ml) was recorded in aqueous crude extract against Bacillus cereus. On the other hand methanol and ethanol crude extracts also showed promising result in the determination of MIC. 50% Ethanol showed the lowest MIC (40 µg/ml) against Staphylococcus aureus, Salmonella species, Staphylococcus hominis. 70% Methanol crude extracts provided the lowest similar MIC (50 µg/ml) against Bacillus cereus and Staphylococcus aureus(Table 6).
Conclusion
Padina gymnospora species used in the present study has a great potential for antimicrobial properties, in addition to cytotoxic activities as well as antioxidant activities under the oxidative stress condition. This study has generated a renewed interest in the possibility of the study sample to be used as a pharmaceutical resource especially their constituents may be applied as drug (antibacterial and anticancer agent) for human administration in different pathogenic condition.
References
- 1. Alejandro, H.B., Carolina, C., Javier, I., et al. Seaweed production: overview of the global state of exploitation, farming and emerging research activity. (2017) European Journal of Phycology 52(4): 391-406.
- 2. Montaser, R., Hendrik, L. Marine natural products: a new wave of drugs? (2011) Future medicinal chemistry ٣(١٢): ١٤٧٥-1489.
- 3. Sarkar, M.S., Kamal, M., Hasan, M.M., et al. Present status of naturally occurring seaweed flora and their utilization in Bangladesh. (2016) Research in Agriculture Livestock and Fisheries 3(1):203-216.
- 4. Saranya, C., Parthiban, C., Anantharaman, P. Evaluation of antibacterial and antioxidant activities of seaweeds from Pondicherry coast. (2014) Advances in Applied Science Research 5(4): 82-90.
PubMed│CrossRef│Others
- 5. Ahmed, H.H., Hegazi, M.M., Fahim, C.B. Cystoseiramyrica and Padinapavonica: A potential natural hope against hepatic injury in animal model. (2016) Der Pharmacia Lettre 8(4):161-172.
PubMed│CrossRef│Others
- 6. Ismail, A., Ktari, L., Ahmed, M., et al. Antimicrobial Activities of Bacteria Associated with the Brown Alga Padinapavonica. (2016) Front Micro biol 7:1072.
- 7. Islam, M.S., Mia, M., Apu, M.A.I., et al. A comprehensive review on region based traditional Ayurvedic practitioner’s plants secondary metabolites and their phytochemical activities in Bangladesh. (2015) Journal of Pharmacognosy and Phytochemistry 3(6): 202-216.
PubMed│CrossRef│Others
- 8. Chander, M.P., Veeraragavam, S., Vijayachari, P. Antimicrobial and Hemolytic activity of seaweed Padinagymnospora from South Andaman, Andaman and Nicobar Islands of India. (2014) International Journal of Microbiology and Applied Sciences 3(6): 364-369.
PubMed│CrossRef│Others
- 9. Elica, A.C.G., Teresinha,G.da.S.,Jaciana, S.A et al. Cytotoxic activity of marine algae against cancerous cells. (2013) Brazilian Journal of Pharmacognosy 23(4): 668-673.
- 10. Tamim, A., Toufuqul, I., Md Abdul, A., et al. Phytochemical screening and evaluation of antioxidant and cytotoxic activities of Halimedaopuntia. (2020) Journal of Marine Biology and Aquaculture 6(1): 1-7.
- 11. Toufiqul, I., Tamim, A., Md Abdul, A., et al. Bioactive compound screening and in vitro appraisal of potential antioxidant and cytotoxicity of Cladophoropsis sp. isolated from the Bay of Bengal. (2020) EC Pharmacology and Toxicology 8(10): 19-31
PubMed│CrossRef│Others
- 12. Ma, C., Yang, K., Wang, Y., et al. Anti-Aging Effectof Agar Oligosaccharide on Male Drosophila melanogaster and its Preliminary Mechanism. (2019) Mar Drugs ١٧(١١): 632.
- 13. Aftab US. Seaweeds of Bangaldesh. (2019) Chittagong, Bangladesh: Institute of Marine Sciences, University of Chittagong.
PubMed│CrossRef│Others
- 14. Gul, R., Jan, S.U., Faridullah, S., et al. Preliminary Phytochemical Screening, Quantitative Analysis of Alkaloids, and Antioxidant Activity of Crude Plant Extracts from Ephedra intermedia Indigenous to Balochistan. (2017) The Scientific World Journal .
- 15. Vimalkumar, C.S., Vilash, V., Krishnakumar, N.M., et al. Comparative Preliminary Phytochemical Analysis of Ethanolic Extracts of leaves of OleaDioicaRoxb, Infected with The Rust fungus Zaghouania oleo (E.J. Butler) Cummins and Non Infected Plants. (2014) J PharmacognPhytochem3(4): 69–72.
PubMed│CrossRef│Others
- 16. Ezeonu, C.S., Ejikeme, C.M. Qualitative and Quantitative Determination of Phytochemical Contents of Indigenous Nigerian Softwoods. (2016) New J Sci 1–9.
- 17. Dahanayake, J.M., Perera, P.K., Galappatty, P., et al. Comparative Phytochemical Analysis and Antioxidant Activities of Tamalakyadi Decoction with Its Modified Dosage Forms. (2019) Evidance Based Complement AlternatMed 6037137.
- 18. Singh, D., Singh, P., Gupta, A., et al. Qualitative estimation of the presence of a bioactive compound in CentellaAsiatica: an important medicinal plant. (2012) Int J Life Sci Med Sci 2(1): 5-7.
- 19. Das, B.K., Das, B., Arpita, F.K., et al. Phytochemical screening and Antioxidant activity of Leucasaspera. (2011) International Journal of Pharmaceutical Sciences and Research 2(7): 1746-1752.
- 20. de Torre, M.P., Cavero, R.Y., Calvo, M.I., et al. A Simple and a Reliable Method to Quantify Antioxidant Activity in Vivo. (2019) Antioxidants (Basel) 8(5): 142.
- 21. Tarik, A., Abdelmjid, A.Brown Seaweed Bifurcariabifurcata: Bioguided Fractionation of Extracts by Antibacterial Activity and Cytotoxicity Test. (2014) Biosciences Biotechnology Research Asia 11(3): 1081-1085.
- 22. Morshed, M.A., Uddin, A., Saifur, R., et al. Evaluation of Antimicrobial and Cytotoxic properties of Leucasaspera and Spilanthespaniculata. (2011) International Journal of Biosciences 1(2): 7-16.
PubMed│CrossRef│Others
- 23. Morshed, M.A., Azim, U., Akhlaqur, R., et al. In vitro Antimicrobial and cytotoxicity screening of Terminalia arjuna ethanol extract. (2011) International Journal of Biosciences 1(2): 31-38.
PubMed│CrossRef│Others
- 24. Morshedul A. Essence of antioxidants in aging science: NRF2, a true fact. (2019) CPQ Medicine 5(5): 1-5.
PubMed│CrossRef│Others
- 25. Clarkson, C., Maharaj, V.J., Crouch, N.R., et al. In vitro antiplasmodial activity of medicinal plants native to or naturalized in South Africa. (2004) J Ethnopharmcol 92(2-3): 177-191.
- 26. El Shoubaky, G.A., Salem, E.A. Terpenes and Sterols Composition of Marine Brown Algae Padinapavonica(Dictyotales) and Hormophysa triquetra(Fucales). (2014) International Journal of Pharmacognosy and Phytochemical Research 6(4): 894-900.
PubMed│CrossRef│Others
- 27. Kamenarskaa, Z., Gasicb, M.J., Zlatovicb, M., et al. Chemical Composition of the Brown Alga Padinapavonia(L.) Gaill. from the Adriatic Sea. (2002) Botanica Marina 45: 339-345.
PubMed│CrossRef│Others
- 28. MdMorshedul, A. Two faces of glucocorticoid receptor (GR) signaling. (2017) EC Pharmacology and Toxicology ECO(1): 22-24.
PubMed│CrossRef│Others
- 29. Yamamoto, M., Kensler, T.W., Motohashi, H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. (2018) Physiol Rev 98(3): 1169-1203.
- 30. Halliwell, B., Gutteridge, J.M., Cross, C.E. Free radicals, antioxidants, and human disease: where are we now? (1992) J Lab Clin Med 119(6): 598–620.
- 31. Antonius,H.C., Yoshihiro, S., Yoshiro, K. Pyropheophytina as an Antioxidative Substance from the Marine Alga, Arame (Eiseniabicyclis). (1992) Bioscience, Biotechnology, and Biochemistry56(10): 1533–1535.
- 32. Ahmed, H.H., Hegazi, M.M., Fahim, C.B. Cystoseiramyrica and Padinapavonica: A potential natural hope against hepatic injury in animal model. (2016) Der Pharmacia Lettre 8(4): 161-172.
PubMed│CrossRef│Others
- 33. Saija, A., Scalese, M., Lanza, M., et al. Flavonoids as antioxidant agents: importance of their interaction with biomembranes. (1995) Free RadicBiol Med 19(4): 481-486.
- 34. Moon, Y.J., Wang, X., Morris, M.E. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. (2006) Toxicolin Vitro 20(2): 187-210.
- 35. Kim, H.P., Son, K.H., Chang, H.W., et al. Anti-inflammatory Plant Flavonoids and Cellular Action Mechanisms. (2004) J PhamacolSci 96(3): 229-245.
- 36. van Acker, S.A., van den Berg, D.J., Tromp, M.N., et al. Structural aspects of antioxidant activity of flavonoids. (1996) Free RadicBiol Med 20(3): 331-342.
- 37. Ebrahimzadeh, M.A., Nabavi, S.M., Nabavi, S.F., et al. Antioxidant activity of Hyoscyamussquarrosus fruits. (2009) Pharmacologyonline 2: 644-650.
PubMed│CrossRef│Others
- 38. Osawa T. Novel natural antioxidants for utilization in food and biological systems Post harvest biochemistry of plant food materials in the tropics. Japan Scientific Societies Press, Tokyo, Japan, 1994 241-251.
PubMed│CrossRef│Others
- 39. Duffy, J.E., Hay, M.E. Seaweed Adaptations to Herbivory. (1990) Bioscience 40(5): 368–375.
- 40. Beuria, T.K., Santra, M.K., Panda D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling.(2005) Biochemistry 44(50): 16584-16593.
- 41. Kelley, C., Zhang, Y., Parhi, A., et al. 3-Phenyl substituted 6, 7-dimethoxyisoquinoline derivatives as FtsZ-targeting antibacterial agents.(2012) Bioorg Med Chem ٢٠(٢٤): ٧٠١٢-٧٠٢٩.












