Successful Treatment of Persistent Biliary Leakage with an Endoprosthetic Transgastric Metal Stent
Andreas Volk , Stefan Brückner, Christoph Reissfelder , Jürgen Hampe, Jürgen Weitz
Affiliation
Department of Visceral, Thoracic and Vascular Surgery, University of Dresden, Dresden, Germany
Corresponding Author
Nuh N. Rahbari M.D., Department of Visceral, Thoracic and Vascular Surgery, University of Dresden, Fetscherstr. 74, 01307 Dresden, Germany, Tel: +49 351 458 19260/ Fax: +49 351 458 4395; E-mail: nuh.rahbari@uniklinikum-dresden.de
Citation
Rahbari, N.N., et al. Successful Treatment of Persistent Biliary Leakage with an Endoprosthetic Transgastric Metal Stent. (2016) J Gastro Dis Liver Func 2(1): 47- 51.
Copy rights
© 2016 Rahbari, N.N. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License.
Abstract
The management of patients with persistent bile leakage after hepatic resection has remained a clinical challenge. Internal drainage with a fully covered metallic stent (Axios) may offer a novel and effective strategy for patients with persistent biliary leakage. Here, we here present a case of the successful treatment of a patient with persistent biliary leakage after a partial hepatectomy with endosonography-guided drainage and the placement of a covered, expandable metal stent (Axios). The presented case underlines the therapeutic complexity of patients with chronic biliary leakage after liver resection. Furthermore, this case serves as a proof-of-principle that internal drainage of a bilioma into the stomach using a metal stent that is subsequently removed may serve as a permanent solution in certain cases. Given some patients’ long histories of various failed various interventions, the identification of patients who are at risk for chronic leakage and the determination of the correct timing for the application of transgastric drainage remain important challenges for the future.
Introduction
Liver resection is an established procedure for various hepatobiliary diseases[1]. Improvements in surgical technique and perioperative management over the past decade have substantially decreased the morbidity and mortality rates[2]. Bile leaks are a major cause of postoperative morbidity and commonly require prolonged hospital stays and repeated diagnostic and therapeutic interventions[3-6]. In previous reports, the incidence of bile leakage after liver resection ranges from 4% to 18%[7]. This variability may be explained by differences in patient populations, the types and extents of procedures, and inconsistent definitions of bile leaks. The introduction of a common definition of post-hepatectomy bile leak is expected to reduce the variability in bile leak rates that has been reported thus far[8,9].
Surgical re-interventions for patients with post-hepatectomy bile leaks can result in significant morbidity and high mortality rates[10]. Within the past two decades, minimally invasive therapeutic interventions for the treatment of bile leakage after liver resection have evolved. In patients without a bilioenteric anastomosis, endoscopic transpapillary interventions for the treatment of bile leaks after liver resection have elicited positive results and are currently considered the treatment of choice[11,12]. Endoscopic sphincterotomy with the insertion of a stent minimizes the resistance caused by the sphincter of Oddi and thus facilitates the drainage of bile into the duodenum. However, in patients with persistent bile leaks following sphincterotomy and stent placement, surgical interventions may still be necessary. Such interventions can include atypical or anatomical resections and the formation of bilioenteric anastomoses depending on the location and output of the bile leak. Due to the technically demanding nature of such reoperations and the potentially high risk of procedure-related complications, the indication for and timing of reoperation should be discussed by an interdisciplinary team that includes surgeons and endoscopists. However, it is well known that in certain patients bile leaks may persist even after reoperation with surgical re-intervention.
In recent years, endosonography with the placement of a covered expandable metal stent has become an established procedure for the treatment of pancreas-related fluid collections or cysts[13]. However, little is known about internal drainage as a therapeutic strategy for patients with biliary collections. Here, we present a case of successful treatment with endosonography- guided drainage with the placement of a covered expandable metal stent (Axios) (AXIOS™, XLUMENA, Mountain View, CA) in a patient with persistent biliary leakage after partial hepatectomy.
Case Presentation
A 64-year-old female patient with recurrent epigastric pain was referred to the medical department for therapy for a newly diagnosed mass and intrahepatic cholangiolithiasis located at the biliary branches of liver segment VI. Laboratory results revealed normal liver enzymes without cholestasis. After endoscopic treatment with sphincterotomy and stent insertion, radiological diagnostics (MRI) could not exclude a cholangio cellular carcinoma. The case was presented to our interdisciplinary visceral board, and due to the endoscopic and radiological findings and potential malignancy, the decision was made to perform a surgical exploration.
Intraoperatively, we performed a cholangiography that found no signs of cholangiolithiasis but did reveal a cystic formation of the right biliary tree and a normal biliary tree on the left side. A right hemi-hepatectomy was performed. The intraoperative course was uneventful. A ‘white test’ with Lipofundin injection through the cystic duct was performed and revealed no bile leakage[14]. A histologic examination of the right liver revealed dilated bile ducts suggestive of Caroli syndrome. After 6 days without pathological findings, the patient presented with elevated infectious parameters without fever. A Computer Tomography (CT) scan of the abdomen revealed a fluid collection with air bubbles at the resection margin. A CT wire-guided drain with bilious output was placed into the fluid collection. The pathogenic agents Enterococcus faecalis and Citrobacter freundii were detected and were treated with imipenem antibiotic. The antibiotic treatment was stopped after ten days.
Due to persistent bile leakage, we performed an ERC three weeks postoperatively and found a leakage originating from the stump of the right hepatic duct. A plastic stent (11.4 F/13 cm) was inserted into the left hepatic duct. After this intervention, a bile leakage with a daily output of 100 ml per day persisted. A second endoscopy was performed, and an additional plastic stent (10 F/9 cm) was placed in the left hepatic duct. However, a bilious output of approximately 50 ml per day persisted and led to a third ERC. In addition to a dilatation of the papillotomy, two straight plastic stents (11.4 F/9 cm) were placed in the common hepatic duct. The CT wire-guided drain was left in place. The patient was discharged after three months (82 days).
Over the next three months, the patient was seen on a regular basis in our outpatient clinic. The output of bilious fluid via the abdominal drain decreased. However, the patient was referred to our department with new-onset abdominal pain and fever. A CT scan revealed a dislocation of the CT wire-guided drain and a small abscess at the resection margin. The abdominal drain was removed, and antibiotic treatment was initiated with Ceftriaxon. Two days later, an ERC was performed and revealed no biliary leakage. Therefore, the two plastic stents were removed. Along with antibiotic treatment, the patient’s discomfort disappeared. A control ultrasound revealed normal sized intrahepatic bile ducts with a small amount of fluid at the resection margin. Based on the ultrasound findings and the clinical course, we removed the stents, and the patient was discharged after 12 days.
One month later, the patient presented again with elevated infectious parameters. A CT scan revealed a recurrent bilioma, and a new CT wire-guided drainage was placed. Antibiotic treatment was not necessary, but the biliary output persisted at approximately 100 ml/day. A percutaneous transhepatic cholangiography (PTC) was not feasible because the bile ducts were not dilated.
After six months had passed since the initial operation, the case was brought before our interdisciplinary visceral board, and based on the assumption that the bile leakage was caused by peripheral bile leakages, the decision was made to perform a second surgical exploration. Intraoperatively, a peripheral bile leakage located in segment IVa was found. An atypical resection of segment IVa was performed. Six days postoperatively, the drainage fluid exhibited elevated bilirubin levels (a fivefold elevation compared with the serum bilirubin level), and the fluid output amount increased. Additionally, the patient developed a wound infection in the midline laparotomy that was treated with daily wound dressings and antibiotics. The abdominal drains that were placed intraoperatively were left in place, and the patient was discharged after 30 days. At the time of discharge, we noted an output of 100 ml bilious fluid/day via the abdominal drains.
One month later, the patient presented with mild fever and an increase in the bilious fluid drainage per day. ACT scan was performed, and an intraabdominal fluid collection was ruled out. Due to the increased bilious output, an ERC was performed and revealed normal biliary drainage from the common hepatic duct (CHD) into the duodenum. Three straight plastic stents (2 x 11.4 F/9 cm, 1 x 10 F/9 cm) were inserted into the CHD. The amount of the biliary output via the abdominal drain decreased, and bilirubin was not detectable in the drainage fluid. The patient was discharged after seven days. A few days later, the patient presented with a new bilioma. ACT-guided drain was placed, and antibiotic treatment (ceftriaxone and metronidazole) was initiated.
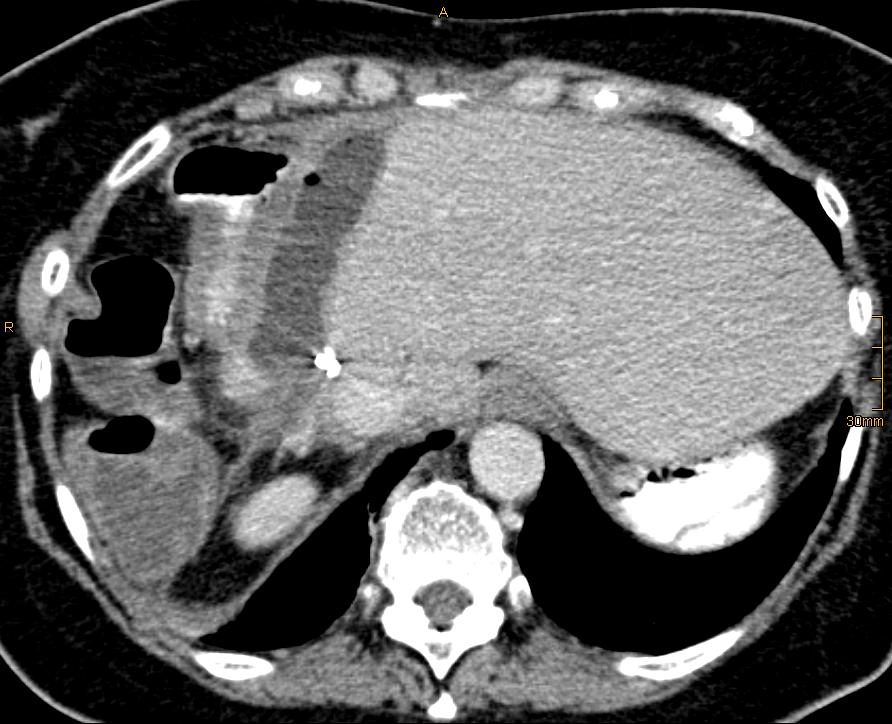
After review of the CT images (Figure. 1) that showed a chronic cavernous formation due to the persistent bile leakage in close proximity to the stomach, a decision in favour of internal drainage into the stomach was made.
Figure 1: CT scan showing the bile leakage in close proximity to the stomach.
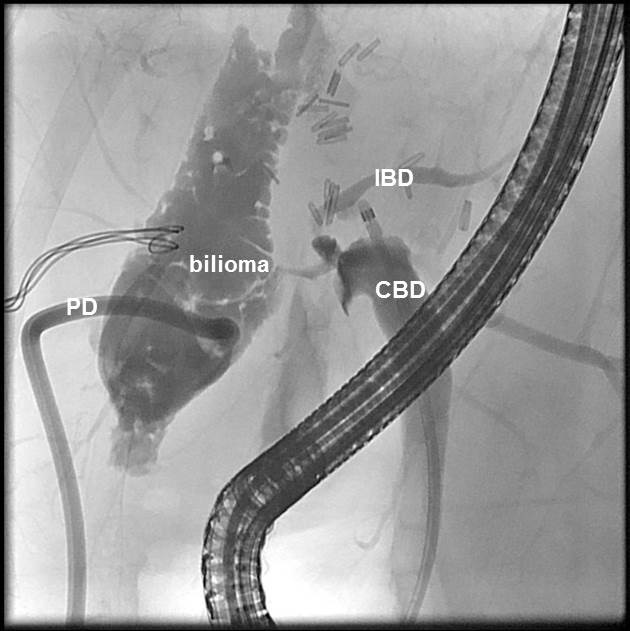
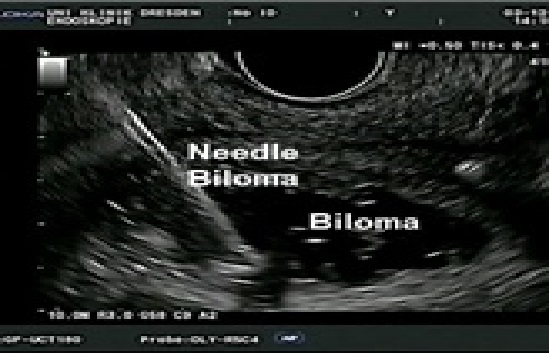
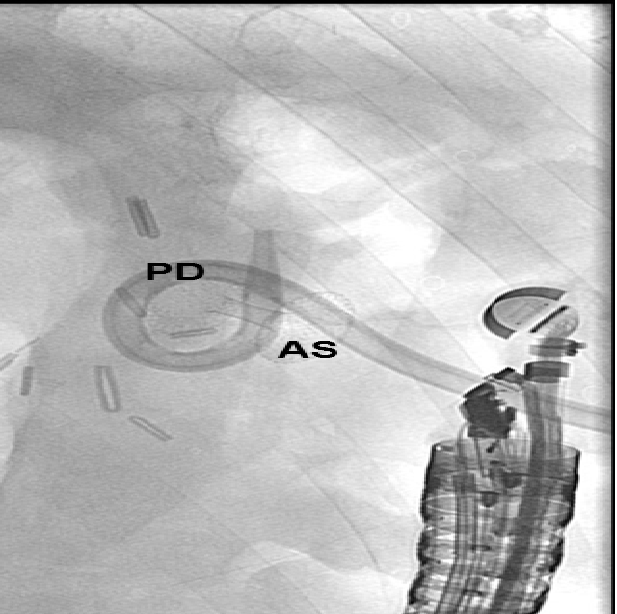
An ERC revealed a persistent bilioma (Figure. 2). To perform drainage, the bilioma was imaged from the gastric antrum using a convex echo endoscope (GF-UGT260, Olympus Optical Co. Ltd., Tokyo, Japan) connected to an ultrasound device (SSD5500; Aloka, Tokyo, Japan). The puncture of the bilioma was performed with a 19-gauge needle (EchoTip, Cook Medical) under real-time ultrasound and colour Doppler imaging guidance (Figure. 3). Iodine contrast medium was injected to obtain fluoroscopic images. After a 0.035-inch guide wire was inserted and positioned, the 19-G needle was removed, and the preloaded AXIOS stent (AXIOS™ XLUMENA, Mountain View, CA) was advanced over the wire into the bilioma using direct electrocautery for the penetration of the wall (AUTOCUT mode, ERBE Electrosurgery, Tübingen Germany) (Figure. 4). The selection of the stent length was determined by the thickness of the interposed tissue. After the secure placement of the stent tip in the bilioma, the distal flange of the stent was deployed under EUS and fluoroscopy guidance followed by proximal traction of the distal flange to place the target lumen in firm opposition to the gastric wall. The proximal flange was then deployed under endoscopic guidance. The stent position was finally verified via endoscopy and fluoroscopy (Figure. 5). During the procedure, the patient was deeply sedated with midazolam and propofol. Endoscopic controls revealed good positioning of the Axios Stent such that the percutaneous drainage could be removed. Three months later, the Axios Stent was removed. Currently, (six months after the removal of the Axios Stent), the patient is in good condition without any sign of recurrent biliary leakage.
Figure 2: Cholangiogram during ERC before the insertion of the AXIOS stent. The common bile duct (CBD) with the catheter inside, intrahepatic bile ducts (IBD), extrabiliary collection of contrast medium (biloma), and the percutaneous drain (PD).
Figure 3: Endoscopic u the bilioma.
Figure 4: AXIOS stent (AS) after the endoscopic-ultrasound guided implantation. The percutaneous drainage (PD) is still in place.
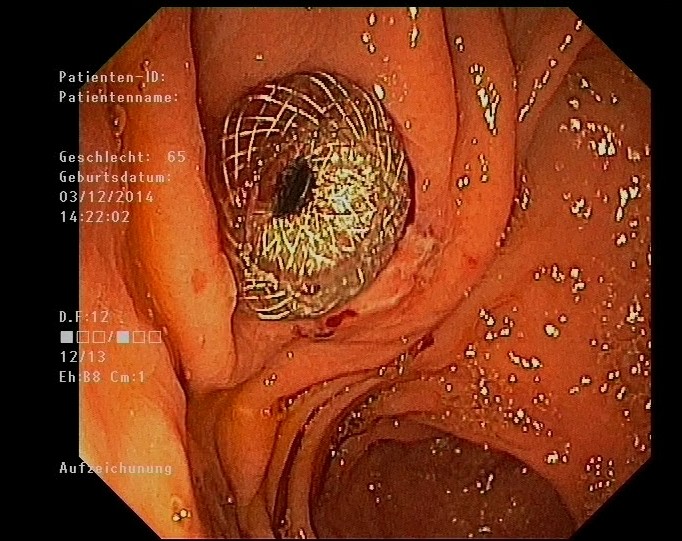
Figure 5: Endoscopic view of the stomach with the AXIOS stent in place.
Discussion
Postoperative bile leakage remains a major source of concern following liver resection due to the associated risks of peritonitis and sepsis. In cases of surgery for malignancy, complications are known to impair long-term prognoses[15,16]. Specifically, chronic bile leakage that persists despite various interventions markedly impairs patient quality of life, delays further treatments and represents a significant source of costs to the health care system. Therefore, safe and effective treatments of (chronic) bile leakage are urgently needed. The majority of studies on the management of bile leakage has addressed the management of post-cholecystectomy bile leakage and has not addressed chronic biliary fistulas. In this scenario, endoscopic interventions, such as biliary sphincterotomy with or without a sphincter-bridging endoprosthesis or an endoprosthesis that span the actual leak site have shown promising results[12,17], although the appropriate endoscopic approach remains a matter of debate[18,19]. Thus, there is currently no consensus regarding the optimal treatment of patients with bile leakage, and patients are commonly treated according to the endoscopist’s discretion. Specifically, the management of patients with persistent bile leakage after hepatic resection has remained a clinical challenge. Internal drainage with a fully covered metallic stent (Axios) may offer a novel and effective strategy for patients with persistent biliary leakage. The AXIOS stent is a fully silicone covered, nitinol braided, self-expanding lumen-apposing metal stent with bilateral anchor flanges. Fully expanded, the flange diameter is twice that of the saddle section and is designed to hold the tissue layers in opposition. Thus far, a small number of cases involving this technique have been described for patients with pancreatic fluid collections[13,20]. Recently, the implantation of this stent has been demonstrated to be feasible for internal drainage of the biliary tree in patients with malignant bile duct obstructions[21]. This type of stent should be chosen when a stable, sealed transluminal conduit needs to be created. This type of stent may offer several advantages over conventional plastic and metal stents in this application due to the lower risks of stent migration and biliary peritonitis and the larger stent diameter. The stent may be removed after 7-8 weeks because the formation of a stable fistula that ensures the continuous drainage of bile may be expected at this time.
However, it should be noted that the experience with this type of procedure remains limited. Although the currently available reports, including the case presented here, suggest low complication rates, further prospective studies are required to fully assess the long-term safety of internal drainage for patients with chronic biliary leakage.
The case presented here underlines the therapeutic complexity of patients with chronic biliary leakage after liver resection. Furthermore, this case serves as a proof-of-principle that the internal drainage of a bilioma into the stomach using a metal stent that is subsequently removed may serve as a permanent solution in certain cases. For patients with long histories of various failed interventions, the identification of patients who are at risk for chronic leakage and the determination of the right timing for the application of transgastric drainage remain important challenges for the future.
Conflict of interest statement:
There are no conflicts of interest.
Supportive foundations:
There are no supportive foundations.
References
- 1. Poon, R.T., Fan, S.T., Lo, C.M., et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. (2004) Ann Surg 240(4): 698-708; discussion -10.
- 2. Rahbari, N.N., Wente, M.N., Schemmer, P., et al. Systematic review and meta-analysis of the effect of portal triad clamping on outcome after hepatic resection. (2008) Br J Surg 95(4): 424-432.
- 3. Lo, C.M., Fan, S.T., Liu, C.L., et al. Biliary complications after hepatic resection: risk factors, management, and outcome. (1998) Arch Surg 133(2):156-161.
- 4. Taguchi, Y., Ebata, T., Yokoyama, Y., et al. The determination of bile leakage in complex hepatectomy based on the guidelines of the International Study Group of Liver Surgery. (2014) World J Surgery 38(1):168-176.
- 5. Reissfelder, C., Rahbari, N.N., Koch, M., et al. Postoperative course and clinical significance of biochemical blood tests following hepatic resection. (2011) Br J Surg 98(6): 836-844.
- 6. Rahbari, N.N., Reissfelder, C., Koch, M., et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. (2011) Ann Surg Oncol 18(13): 3640-3649.
- 7. Erdogan, D., Busch, O.R., van Delden, O.M., et al. Incidence and management of bile leakage after partial liver resection. (2008) Dig Surg 25(1): 60-66.
- 8. Koch, M., Garden, O.J., Padbury, R., et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. (2011) Surgery 149(5): 680-688.
- 9. Rahbari, N.N., Elbers, H., Koch, M., et al. Bilirubin level in the drainage fluid is an early and independent predictor of clinically relevant bile leakage after hepatic resection. (2012) Surgery 152(5): 821-831.
- 10. Pace, R.F., Blenkharn, J.I., Edwards, W.J., et al. Intra-abdominal sepsis after hepatic resection. (1989) Ann Surg 209(3): 302-306.
- 11. Nuzzo, G., Giuliante, F., Giovannini, I., et al. Advantages of multidisciplinary management of bile duct injuries occurring during cholecystectomy. (2008) Am J Surg 195(6): 763-769.
- 12. Donnellan, F., Zeb, F., Courtney, G., et al. Successful outcome of sphincterotomy and 7 French pigtail stent insertion in the management of post-cholecystectomy bile leaks. (2009) Hepatobiliary Pancreat Dis Int 8(3): 309-311.
- 13. Gornals, J.B., De la Serna-Higuera, C., Sanchez-Yague, A., et al. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. (2013) Surg Endosc 27(4): 1428-1434.
- 14. Linke, R., Ulrich, F., Bechstein, W.O., et al. The White-test helps to reduce biliary leakage in liver resection: a systematic review and meta-analysis. (2015) Ann Hepatol 14(2): 161-167.
- 15. Schiesser, M., Chen, J.W., Maddern, G.J., et al. Perioperative morbidity affects long-term survival in patients following liver resection for colorectal metastases. (2008) J Gastrointest Surg 12(6): 1054-1060.
- 16. Farid, S.G., Aldouri, A., Morris-Stiff, G., et al. Correlation between postoperative infective complications and long-term outcomes after hepatic resection for colorectal liver metastasis. (2010) Ann Surg 251(1): 91-100.
- 17. Zerem, E., Omerovic, S. Minimally invasive management of biliary complications after laparoscopic cholecystectomy. (2009) Eur J Intern Med 20(7): 686-689.
- 18. Simmons, D.T., Petersen, B.T., Gostout, C.J., et al. Risk of pancreatitis following endoscopically placed large-bore plastic biliary stents with and without biliary sphincterotomy for management of postoperative bile leaks. (2008) Surg Endosc 22(6): 1459-1463.
- 19. Freeman, M.L., Nelson, D.B., Sherman, S., et al. Complications of endoscopic biliary sphincterotomy. (1996) N Engl J Med 335(13): 909-918.
- 20. Shah, R.J., Shah, J.N., Waxman, I., et al. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. (2015) Clin Gastroenterol Hepatol 13(4): 747-752.
- 21. Bruckner, S., Arlt, A., Hampe, J. Endoscopic ultrasound-guided biliary drainage using a lumen-apposing self-expanding metal stent: a case series. (2015) Endoscopy 47(9): 858-861.